Abstract
Purpose
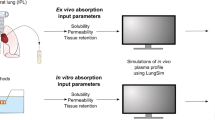
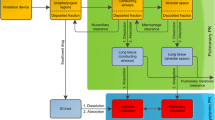
For optimizing the local, pulmonary targeting of inhaled medications, it is important to analyze the relationship between the physicochemical properties of small molecules and their absorption, retention and distribution in the various cell types of the airways and alveoli.
Methods
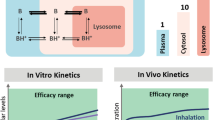
A computational, multiscale, cell-based model was constructed to facilitate analysis of pulmonary drug transport and distribution. The relationship between the physicochemical properties and pharmacokinetic profile of monobasic molecules was explored. Experimental absorption data of compounds with diverse structures were used to validate this model. Simulations were performed to evaluate the effect of active transport and organelle sequestration on the absorption kinetics of compounds.
Results
Relating the physicochemical properties to the pharmacokinetic profiles of small molecules reveals how the absorption half-life and distribution of compounds are expected to vary in different cell types and anatomical regions of the lung. Based on logP, pKa and molecular radius, the absorption rate constants (Ka) calculated with the model were consistent with experimental measurements of pulmonary drug absorption.
Conclusions
The cell-based mechanistic model developed herein is an important step towards the rational design of local, lung-targeted medications, facilitating the design and interpretation of experiments aimed at optimizing drug transport properties in lung.








Similar content being viewed by others
REFERENCES
Brewis RL, Corrin B, Geddes DM, Gibson GJ, editors. Respiratory medicine. London: WB Saunders; 1995.
Ehrhardt C, Kim K-J, editors. Drug absorption studies: in situ, in vitro and in silico models. Arlington: Springer; 2008.
Lipworth BJ. Pharmacokinetics of inhaled drugs. Br J Clin Pharmacol. 1996;42:697–705.
Wall DA, Lanutti AT. High-levels of exopeptidase activity are present in rat and canine bronchoalveolar lavage fluid. Int J Pharm. 1993;97:171–81.
Patton JS. Mechanisms of macromolecule absorption by the lungs. Adv Drug Deliver Rev. 1996;19:3–36.
Anttila S, Hukkanen J, Hakkola J, Stjernvall T, Beaune P, Edwards RJ, et al. Expression and localization of CYP3A4 and CYP3A5 in human lung. Am J Resp Cell Mol. 1997;16:242–9.
Niven RW. Delivery of biotherapeutics by inhalation aerosol. Crit Rev Ther Drug. 1995;12:151–231.
LiCalsi C, Christensen T, Bennett JV, Phillips E, Witham C. Dry powder inhalation as a potential delivery method for vaccines. Vaccine. 1999;17:1796–803.
Russell KE, Read MS, Bellinger DA, Leitermann K, Rup BJ, McCarthy KP, et al. Intratracheal administration of recombinant human factor IX (BeneFix) achieves therapeutic levels in hemophilia B dogs. Thromb Haemost. 2001;85:445–9.
Skyler JS, Cefalu WT, Kourides IA, Landschulz WH, Balagtas CC, Cheng SL, et al. Efficacy of inhaled human insulin in type 1 diabetes mellitus: a randomised proof-of-concept study. Lancet. 2001;357:331–5.
Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6:67–74.
Gumbleton M, Taylor G. Challenges and innovations in effective pulmonary systemic and macromolecular drug delivery. Adv Drug Deliv Rev. 2006;58:993–5.
Shoyele SA, Cawthorne S. Particle engineering techniques for inhaled biopharmaceuticals. Adv Drug Deliv Rev. 2006;58:1009–29.
Yu LX, Lipka E, Crison JR, Amidon GL. Transport approaches to the biopharmaceutical design of oral drug delivery systems: prediction of intestinal absorption. Adv Drug Deliver Rev. 1996;19:359–76.
Egan WJ, Lauri G. Prediction of intestinal permeability. Adv Drug Deliver Rev. 2002;54:273–89.
Norinder U, Haeberlein M. Computational approaches to the prediction of the blood-brain distribution. Adv Drug Deliver Rev. 2002;54:291–313.
Brown RA, Schanker LS. Absorption of aerosolized drugs from the rat lung. Drug Metab Dispos. 1983;11:355–60.
Schanker LS, Hemberger JA. Relation between molecular-weight and pulmonary absorption rate of lipid-insoluble compounds in neonatal and adult-rats. Biochem Pharmacol. 1983;32:2599–601.
Schanker LS, Mitchell EW, Brown RA. Species comparison of drug absorption from the lung after aerosol inhalation or intratracheal injection. Drug Metab Dispos. 1986;14:79–88.
Effros RM, Pornsuriyasak P, Porszasz J, Casaburi R. Indicator dilution measurements of extravascular lung water: basic assumptions and observations. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1023–1031.
Effros RM. Exhaled breath condensates and COPD. Eur Respir J. 2009;33:1238.
Tronde A. Pulmonary drug absorption: in vitro and in vivo investigations of drug absorption across the lung barrier and its relation to drug physicochemical properties, Faculty of Pharmacy, Vol. Ph.D. Uppsala University; 2002.
Tronde A, Norden B, Jeppsson AB, Brunmark P, Nilsson E, Lennernas H, et al. Drug absorption from the isolated perfused rat lung—correlations with drug physicochemical properties and epithelial permeability. J Drug Target. 2003;11:61–74.
Tronde A, Norden B, Marchner H, Wendel AK, Lennernas H, Bengtsson UH. Pulmonary absorption rate and bioavailability of drugs in vivo in rats: structure–absorption relationships and physicochemical profiling of inhaled drugs. J Pharm Sci. 2003;92:1216–33.
Manford F, Tronde A, Jeppsson AB, Patel N, Johansson F, Forbes B. Drug permeability in 16HBE14o—airway cell layers correlates with absorption from the isolated perfused rat lung. Eur J Pharm Sci. 2005;26:414–20.
Oprea TI. Chemical space navigation in lead discovery. Curr Opin Chem Biol. 2002;6:384–9.
Zhang X, Shedden K, Rosania GR. A cell-based molecular transport simulator for pharmacokinetic prediction and cheminformatic exploration. Mol Pharmaceut. 2006;3:704–16.
Zhang X, Zheng N, Rosania GR. Simulation-based cheminformatic analysis of organelle-targeted molecules: lysosomotropic monobasic amines. J Comput Aided Mol Des. 2008;22:629–45.
Trapp S, Horobin RW. A predictive model for the selective accumulation of chemicals in tumor cells. Eur Biophys J. 2005;34:959–66.
Balon K, Riebesehl BU, Muller BW. Drug liposome partitioning as a tool for the prediction of human passive intestinal absorption. Pharm Res. 1999;16:882–8.
Parent RA, editor. Treatise on pulmonary toxicology: comparative biology of the normal lung. Boca Raton: CRC; 1992.
Widdicombe JG. Airway liquid: a barrier to drug diffusion? Eur Respir J. 1997;10:2194–7.
Salmon M, Walsh DA, Huang TJ, Barnes PJ, Leonard TB, Hay DW, et al. Involvement of cysteinyl leukotrienes in airway smooth muscle cell DNA synthesis after repeated allergen exposure in sensitized Brown Norway rats. Br J Pharmacol. 1999;127:1151–8.
Cohen MD, Zelikoff JT, Schlesinger RB, editors. Pulmonary immunotoxicology. New York: Springer; 2000.
Crane GJ, Kotecha N, Luff SE, Neil TO. Electrical coupling between smooth muscle and endothelium in arterioles of the guinea-pig small intestine. Phys Med Biol. 2001;46:2421–34.
James AL, Bai TR, Mauad T, Abramson MJ, Dolhnikoff M, McKay KO, et al. Airway smooth muscle thickness in asthma is related to severity but not duration of asthma. Eur Respir J. 2009;34(5):1040–45.
Yeh HC, Schum GM, Duggan MT. Anatomic models of the tracheobronchial and pulmonary regions of the rat. Anat Rec. 1979;195:483–92.
Miller LA, Hurst SD, Coffman RL, Tyler NK, Stovall MY, Chou DL, et al. Airway generation-specific differences in the spatial distribution of immune cells and cytokines in allergen-challenged rhesus monkeys. Clin Exp Allergy. 2005;35:894–906.
Ross MH, Pawlina W, editors. Histology: a text and Atlas: with correlated cell and molecular biology. Baltimore: Lippincott Williams & Wilkins; 2006.
Kroll F, Karlsson JA, Persson CG. Tracheobronchial microvessels perfused via the pulmonary artery in guinea-pig isolated lungs. Acta Physiol Scand. 1987;129:445–6.
Sims DE, Horne MM. Heterogeneity of the composition and thickness of tracheal mucus in rats. Am J Physiol. 1997;273:L1036–1041.
Veldhuizen R, Nag K, Orgeig S, Possmayer F. The role of lipids in pulmonary surfactant. Biochim Biophys Acta. 1998;1408:90–108.
Doppenschmitt S, Spahn-Langguth H, Regardh CG, Langguth P. Role of P-glycoprotein-mediated secretion in absorptive drug permeability: an approach using passive membrane permeability and affinity to P-glycoprotein. J Pharm Sci. 1999;88:1067–72.
Troutman MD, Thakker DR. Efflux ratio cannot assess P-glycoprotein-mediated attenuation of absorptive transport: asymmetric effect of P-glycoprotein on absorptive and secretory transport across Caco-2 cell monolayers. Pharm Res. 2003;20:1200–9.
Tubic M, Wagner D, Spahn-Langguth H, Bolger MB, Langguth P. In silico modeling of non-linear drug absorption for the P-gp substrate talinolol and of consequences for the resulting pharmacodynamic effect. Pharm Res. 2006;23:1712–20.
Shirasaka Y, Sakane T, Yamashita S. Effect of P-glycoprotein expression levels on the concentration-dependent permeability of drugs to the cell membrane. J Pharm Sci. 2008;97:553–65.
Frenchand MC, Wishart GN. Isolated perfused rabbit lung as a model to study the absorption of organic aerosols. J Pharmacol Method. 1985;13:241–8.
Hamilton KO, Backstrom G, Yazdanian MA, Audus KL. P-glycoprotein efflux pump expression and activity in Calu-3 cells. J Pharm Sci. 2001;90:647–58.
Hamilton KO, Topp E, Makagiansar I, Siahaan T, Yazdanian M, Audus KL. Multidrug resistance-associated protein-1 functional activity in Calu-3 cells. J Pharmacol Exp Ther. 2001;298:1199–205.
Endter S, Francombe D, Ehrhardt C, Gumbleton M. RT-PCR analysis of ABC, SLC and SLCO drug transporters in human lung epithelial cell models. J Pharm Pharmacol. 2009;61:583–91.
Wagner D, Glube N, Berntsen N, Tremel W, Langguth P. Different dissolution media lead to different crystal structures of talinolol with impact on its dissolution and solubility. Drug Dev Ind Pharm. 2003;29:891–902.
Marvin and Calculator, ChemAxon, Budapest, Hungary, 2007.
Petitjean M. Applications of the radius diameter diagram to the classification of topological and geometrical shapes of chemical-compounds. J Chem Inf Comp Sci. 1992;32:331–7.
MOE: Molecular Operating Environment, Chemical Computing Group, Montreal, Quebec, Canada, 2007.
ACKNOWLEDGEMENTS
This work was supported by NIH Grant RO1GM078200 to G. Rosania, and by a Fred Lyons Jr. Fellowship from the College of Pharmacy at University of Michigan to J. Yu. The authors thank Newaj Abdullah for collecting lung-relevant parameters.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 860 kb)
Rights and permissions
About this article
Cite this article
Yu, Jy., Rosania, G.R. Cell-Based Multiscale Computational Modeling of Small Molecule Absorption and Retention in the Lungs. Pharm Res 27, 457–467 (2010). https://doi.org/10.1007/s11095-009-0034-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-0034-9