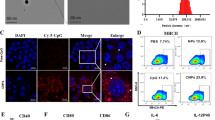
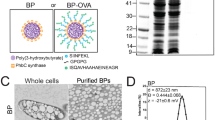
ABSTRACT
Purpose
To develop a novel cancer vaccine using the targeting system of antigen protein to antigen-presenting cells (APCs) for efficient and safe cancer therapy.
Methods
The novel delivery system was constructed with antigen protein, benzalkonium chloride (BK), and γ-polyglutamic acid (γ-PGA), using ovalbumin (OVA) as a model antigen protein and evaluating its immune induction effects and utilities for cancer vaccine.
Results
BK and γ-PGA enabled encapsulation of OVA and formed stable anionic particles at nanoscale, OVA/BK/γ-PGA complex. Complex was taken up by dendritic cell line DC2.4 cells efficiently. We subcutaneously administered the complex to mice and examined induction of IgGs. The complex induced not only Th2-type immunoglobulins but also Th1-type immunoglobulins. OVA/BK/γ-PGA complex inhibited tumor growth of E.G7 cells expressing OVA regularly; administered OVA/BK/γ-PGA complex completely rejected tumor cells.
Conclusion
The novel vaccine could be platform technology for a cancer vaccine.







Similar content being viewed by others
REFERENCES
Tan A, De La Peña H, Seifalian AM. The application of exosomes as a nanoscale cancer vaccine. Int J Nanomed. 2010;5:889–900.
Andersen MH, Junker N, Ellebaek E, Svane IM, Thor Straten P. Therapeutic cancer vaccines in combination with conventional therapy. J Biomed Biotechnol. 2010;2010(237623):1–10.
Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117.
Kyte JA, Trachsel S, Risberg B, thor Straten P, Lislerud K, Gaudernack G. Unconventional cytokine profiles and development of T cell memory in long-term survivors after cancer vaccination. Canc Immunol Immunother. 2009;58(10):1609–26.
Zimmermann VS, Casati A, Schiering C, Caserta S, Hess Michelini R, Basso V, et al. Tumors hamper the immunogenic competence of CD4+ T cell-directed dendritic cell vaccination. J Immunol. 2007;179(5):2899–909.
McKee AS, Munks MW, Marrack P. How do adjuvants work? Important considerations for new generation adjuvants. Immunity. 2007;27(5):687–90.
Dubensky Jr TW, Reed SG. Adjuvants for cancer vaccines. Semin Immunol. 2010;22(3):155–61.
Asif M, Jenkins KA, Hilton LS, Kimpton WG, Bean AG, Lowenthal JW. Cytokines as adjuvants for avian vaccines. Immunol Cell Biol. 2004;82(6):638–43.
Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65(20):3231–40.
Ulmer JB, Wahren B, Liu MA. Gene-based vaccines: recent technical and clinical advances. Trends Mol Med. 2006;12(5):216–22.
Kurosaki T, Kitahara T, Fumoto S, Nishida K, Nakamura J, Niidome T, et al. Ternary complexes of pDNA, polyethylenimine, and gamma-polyglutamic acid for gene delivery systems. Biomaterials. 2009;30(14):2846–53.
Kurosaki T, Kitahara T, Kawakami S, Nishida K, Nakamura J, Teshima M, et al. The development of a gene vector electrostatically assembled with a polysaccharide capsule. Biomaterials. 2009;30(26):4427–34.
Shuaibu MN, Cherif MS, Kurosaki T, Helegbe GK, Kikuchi M, Yanagi T, et al. Effect of nanoparticle coating on the immunogenicity of plasmid DNA vaccine encoding P. yoelii MSP-1 C-terminal. Vaccine. 2011;29(17):3239–47.
Yoshikawa T, Okada N, Oda A, Matsuo K, Matsuo K, Kayamuro H, et al. Nanoparticles built by self-assembly of amphiphilic gamma-PGA can deliver antigens to antigen-presenting cells with high efficiency: a new tumor-vaccine carrier for eliciting effector T cells. Vaccine. 2008;26(10):1303–13.
Janikashvili N, Larmonier N, Katsanis E. Personalized dendritic cell-based tumor immunotherapy. Immunotherapy. 2010;2(1):57–68.
Palucka K, Ueno H, Fay J, Banchereau J. Dendritic cells and immunity against cancer. J Intern Med. 2011;269(1):64–73.
Neyestani TR, Woodward WD, Hillyer L. Serum levels of Th2-Type immunoglobulins are increased in weanling mice subjected to acute wasting protein-energy malnutrition. Iran J Allergy Asthma Immunol. 2004;3(1):1–6.
Prodhomme EJ, Tutt AL, Glennie MJ, Bugg TD. Multivalent conjugates of poly-gamma-D-glutamic acid from Bacillus licheniformis with antibody F(ab′) and glycopeptide ligands. Bioconjug Chem. 2003;14(6):1148–55.
Ye H, Jin L, Hu R, Yi Z, Li J, Wu Y, et al. Poly(gamma, L-glutamic acid)-cisplatin conjugate effectively inhibits human breast tumor xenografted in nude mice. Biomaterials. 2006;27(35):5958–65.
Marple B, Roland P, Benninger M. Safety review of benzalkonium chloride used as a preservative in intranasal solutions: an overview of conflicting data and opinions. Otolaryngol Head Neck Surg. 2004;130(1):131–41.
Liebert MA. Final report on the safety assessment of benzalkonium chloride. Int J Toxicol. 1989;8(4):589–625.
ACKNOWLEDGMENTS & DISCLOSURES
This study was supported in part by the Uehara Memorial Foundation, the Global COE Program, Nagasaki University, Japan, Grant-in-Aid for JSPS Fellows, and Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurosaki, T., Kitahara, T., Nakamura, T. et al. Development of Effective Cancer Vaccine Using Targeting System of Antigen Protein to APCs. Pharm Res 29, 483–489 (2012). https://doi.org/10.1007/s11095-011-0571-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0571-x