Abstract
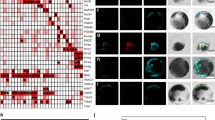
Nearest neighbor analysis of immunocytolocalization experiments indicates that the enzymes glyceraldehyde-3-P dehydrogenase, triose-P isomerase and aldolase are located close to one another in the pea leaf chloroplast stroma, and that aldolase is located close to sedoheptulose bisphosphatase. Direct transfer of the triose phosphates between glyceraldehyde-3-P dehydrogenase and triose-P isomerase, and from glyceraldehyde-3-P dehydrogenase and triose-P isomerase to aldolase, is then a possibility, as is direct transfer of sedoheptulose bisphosphate from aldolase to sedoheptulose bisphosphatase. Spatial organization of these enzymes may be important for efficient CO2 fixation in photosynthetic organisms. In contrast, there is no indication that fructose bisphosphatase is co-localized with aldolase, and direct transfer of fructose bisphosphate from aldolase to fructose bisphosphatase seems unlikely.
Similar content being viewed by others
References
JB Anderson AA Carol VK Brown LE Anderson (2003) ArticleTitleA quantitative method for assessing co-localization in immuno-labeled thin section electron micrographs J Struct Biol 143 95–106 Occurrence Handle10.1016/S1047-8477(03)00138-2 Occurrence Handle12972346
LE Anderson (1971) ArticleTitleChloroplast and cytoplasmic enzymes. II. Pea leaf triose phosphate isomerases Biochim Biophys Acta 235 237–244 Occurrence Handle5089710
LE Anderson AA Carol (2004a) ArticleTitleEnzyme co-localization with Rubisco in pea leaf chloroplasts Photosynth Res 82 49–58 Occurrence Handle10.1023/B:PRES.0000040443.92346.37
LE Anderson AA Carol (2004b) ArticleTitleSeven enzymes of carbon metabolism, including three Calvin cycle isozymes, are present in the secondary cell wall thickenings of the developing xylem tracheary elements in pea leaves Int J Plant Sci 165 243–256 Occurrence Handle10.1086/381918
LE Anderson IM Goldhaber-Gordon D Li X-Y Tang M Xiang N Prakash (1995a) ArticleTitleEnzyme-enzyme interaction in the chloroplast: glyceraldehyde-3-phosphate dehydrogenase, triose phosphate isomerase and aldolase Planta 196 245–255
LE Anderson X Wang JT Gibbons (1995b) ArticleTitleThree enzymes of carbon metabolism, or their antigenic analogs, in pea nuclei Plant Physiol 108 659–667 Occurrence Handle10.1104/pp.108.2.659
LE Anderson JT Gibbons X Wang (1996) ArticleTitleDistribution of ten enzymes of carbon metabolism in pea (Pisum sativum) chloroplasts Int J Plant Sci 157 525–538 Occurrence Handle10.1086/297372
LE Anderson R Yousefzai MR Ringenberg AA Carol (2004) ArticleTitleBoth chloroplastic and cytosolic fructose bisphosphatase isozymes are present in the pea leaf nucleus Plant Sci 166 721–730 Occurrence Handle10.1016/j.plantsci.2003.11.008
MP Babadzhanova MA Babadzhanova KA Aliev (2002) ArticleTitleFree and membrane-bound multienzyme complexes with Calvin cycle activities in cotton leaves Russian J Plant Physiol 49 592–597 Occurrence Handle10.1023/A:1020272414760
S Beeckmans van Driessche L Kanarek (1990) ArticleTitleClustering of sequential enzymes in the glycolytic pathway and the citric acid cycle J Cell Biochem 43 297–306 Occurrence Handle10.1002/jcb.240430402 Occurrence Handle2398101
KH Choi DR Tolan (2004) ArticleTitlePresteady-state kinetic evidence for a ring-opening activity in fructose-1,6-(bis)phosphate aldolase J Am Chem Soc 126 3402–3403 Occurrence Handle10.1021/ja038540u Occurrence Handle15025449
A Chueca M Sahrawy EA Pagano JL Gorge (2002) ArticleTitleChloroplast fructose-1,6-bisphosphatase: structure and function Photosynth Res 74 235–249 Occurrence Handle10.1023/A:1021243110495
D Dziewulska-Szwajkowska M Zmojdzian P Dobryszycki M Kochman A Dzugaj (2004) ArticleTitleThe interaction of FBPase with aldolase: a kinetic and fluorescence investigation on chicken muscle enzymes Comp Biochem Physiol B Biochem Mol Biol 137 115–129 Occurrence Handle10.1016/j.cbpc.2003.10.010 Occurrence Handle14698918
JS Easterby (1981) ArticleTitleA generalized theory of the transition time for sequential enzyme reactions Biochem J 199 155–161 Occurrence Handle7337699
AH Elcock GA Huber JA McCammon (1997) ArticleTitleElectrostatic channeling of substrates between enzyme active sites: comparison of simulation and experiment Biochemistry 36 16049–16058 Occurrence Handle10.1021/bi971709u Occurrence Handle9405038
R Fishbein PA Benkovic KJ Schray IJ Siewers JJ Steffens SJ Benkovic (1974) ArticleTitleAnomeric specificity of phosphofructokinase from rabbit muscle J Biol Chem 249 6047–6051 Occurrence Handle4278654
WA Frey R Fishbein Maine MM de SJ Benkovic (1977) ArticleTitleSubstrate form of D-frutose 1,6-bisphosphate utilized by fructose 1,6-bisphosphatase Biochemistry 16 2479–2484 Occurrence Handle10.1021/bi00630a025 Occurrence Handle193561
F Gavilanes C Salerno P Fasella (1981) ArticleTitleHeterologous enzyme–enzyme complex between D-fructose-1,6-bisphosphate aldolase and triosephosphate isomerase from Ceratitis capitata Biochim Biophys Acta 660 154–156 Occurrence Handle7272313
T Gefflaut C Blonski J Perie M Willson (1995) ArticleTitleClass I aldolases: substrate specificity, mechanism, inhibitors and structural aspects Prog Biophys Mol Biol 63 301–340 Occurrence Handle10.1016/0079-6107(95)00008-9 Occurrence Handle8599032
B Gontero ML Cárdenas J Ricard (1988) ArticleTitleA functional five-enzyme complex of chloroplasts involved in the Calvin cycle Eur J Biochem 173 437–443 Occurrence Handle10.1111/j.1432-1033.1988.tb14018.x Occurrence Handle2834208
E Graciet S Lebreton B Gontero (2004) ArticleTitleEmergence of new regulatory mechanisms in the Benson–Calvin pathway via protein-protein interactions: a glyceraldehyde-3-phosphate dehydrogenase/CP12/phosphoribulokinase complex J Exp Bot 55 1245–1254 Occurrence Handle10.1093/jxb/erh107 Occurrence Handle15047759
V Haake R Zrenner U Sonnewald M Stitt (1998) ArticleTitleA moderate decrease of plastid aldolase activity inhibits photosynthesis, alters the levels of sugars and starch, and inhibits growth of potato plants Plant J 14 147–157 Occurrence Handle10.1046/j.1365-313X.1998.00089.x Occurrence Handle9628012
V Haake M Geiger Pia Walch-Liu C Engels R Zrenner M Stitt (1999) ArticleTitleChanges in aldolase activity in wild-type potato plants are important for acclimation to growth irradiance and carbon dioxide concentration, because plastid aldolase exerts control over the ambient rate of photosynthesis across a range of growth conditions Plant J 17 479–489 Occurrence Handle10.1046/j.1365-313X.1999.00391.x
JA Jebanathirajah JR Coleman (1998) ArticleTitleAssociation of carbonic anhydrase with a Calvin cycle enzyme complex in Nicotiana tabacum Planta 204 177–182 Occurrence Handle10.1007/s004250050244 Occurrence Handle9487724
RB Kachru LE Anderson (1975) ArticleTitleInactivation of pea leaf phosphofructokinase by light and dithiothreitol Plant Physiol 55 199–202
MF Liaud C Lichtle K Apt W Martin R Cerff (2000) ArticleTitleCompartment-specific isoforms of TPI and GAPDH are imported into diatom mitochondria as a fusion protein: evidence in favor of a mitochondrial origin of the eukaryotic glycolytic pathway Mol Biol Evol 17 213–223 Occurrence Handle10677844
JS MacGregor VN Singh S Davoust E Melloni S Pontremoli BL Horecker (1980) ArticleTitleEvidence for formation of a rabbit liver aldolase – rabbit liver fructose-1,6-bisphosphatase complex Proc Natl Acad Sci U S A 77 3889–3892 Occurrence Handle6253999
IA Marques DM Ford G Muschinek LE Anderson (1987) ArticleTitlePhotosynthetic carbon metabolism in isolated pea chloroplasts: metabolite levels and enzyme activities Arch Biochem Biophys 252 458–466 Occurrence Handle10.1016/0003-9861(87)90052-X Occurrence Handle3813547
P Mendes DB Kell HV Westerhoff (1992) ArticleTitleChannelling can decrease pool size Eur J Biochem 204 257–266 Occurrence Handle10.1111/j.1432-1033.1992.tb16632.x Occurrence Handle1740137
P Mendes DB Kell HV Westerhoff (1996) ArticleTitleWhy and when channelling can decrease pool size at constant net flux in a simple dynamic channel Biochim Biophys Acta 1289 175–186 Occurrence Handle8600971
GB Moorhead RJ Hodgson WC Plaxton (1994) ArticleTitleCopurification of cytosolic fructose-1,6-bisphosphatase and cytosolic aldolase from endosperm of germinating castor oil seeds Arch Biochem Biophys 312 326–335 Occurrence Handle10.1006/abbi.1994.1316 Occurrence Handle8037444
F Orosz J Ovadi (1986) ArticleTitleDynamic interactions of enzymes involved in triosephosphate metabolism Eur J Biochem 160 615–619 Occurrence Handle10.1111/j.1432-1033.1986.tb10082.x Occurrence Handle3780725
S Pontremoli E Melloni F Salamino B Sparatore M Michetti VN Singh BL Horecker (1979) ArticleTitleEvidence for an interaction between fructose 1,6-bisphosphatase and fructose 1,6-bisphosphate aldolase Arch Biochem Biophys 197 356–363 Occurrence Handle10.1016/0003-9861(79)90256-X Occurrence Handle232404
J Qi MN Isupov JA Littlechild LE Anderson (2001) ArticleTitleChloroplast glyceraldehyde-3-P dehydrogenase contains a single disulfide bond located in the C-terminal extension to the B subunit J Biol Chem 276 35247–35252 Occurrence Handle10.1074/jbc.M103855200 Occurrence Handle11438534
D Rakus P Mamczur A Gizak D Dus A Dzugaj (2003) ArticleTitleColocalization of muscle FBPase and muscle aldolase on both sides of the Z-line Biochem Biophys Res Commun 311 294–299 Occurrence Handle10.1016/j.bbrc.2003.09.209 Occurrence Handle14592412
D Rakus M Pasek H Krotkiewski A Dzugaj (2004) ArticleTitleInteraction between muscle aldolase and muscle fructose 1,6-bisphosphatase results in the substrate channeling Biochemistry 43 14948–14957 Occurrence Handle10.1021/bi048886x Occurrence Handle15554702
K Razdan RL Heinrikson H Zurcher-Neely PW Morris LE Anderson (1992) ArticleTitleChloroplast and cytoplasmic enzymes: isolation and sequencing of cDNAs coding for two distinct pea chloroplast aldolases Arch Biochem Biophys 298 192–197 Occurrence Handle10.1016/0003-9861(92)90112-A Occurrence Handle1524427
SJ Reynolds DW Yates CI Pogson (1971) ArticleTitleDihydroxyacetone phosphate. Its structure and reactivity with α-glycerophosphate dehydrogenase aldolase and triose phosphate isomerase and some possible metabolic implications Biochem J 122 285–297 Occurrence Handle4330197
C Salerno J Ovadi (1982) ArticleTitleInteraction between D-fructose-1,6-bisphosphate aldolase and triosephosphate isomerase. Mutual protection against perchloric acid denaturation FEBS Lett 138 270–272 Occurrence Handle10.1016/0014-5793(82)80458-4 Occurrence Handle6279447
JK Sainis Harris GC (1986) ArticleTitleThe association of ribulose-1,5-bisphosphate carboxylase with phosphoriboisomerase and phosphoribulokinase Biochem Biophys Res Commun 139 947–954 Occurrence Handle10.1016/S0006-291X(86)80269-8 Occurrence Handle3021158
KJ Schray R Fishbein WP Bullard SJ Benkovic (1975) ArticleTitleThe anomeric form of D-fructose 1,6-bisphosphate used as substrate in the muscle and yeast aldolase reactions J Biol Chem 250 4883–4887 Occurrence Handle168195
P Stephan F Clarke D Morton (1986) ArticleTitleThe indirect binding of triose-phosphate isomerase to myofibrils to form a glycolytic enzyme mini-complex Biochim Biophys Acta 873 127–135 Occurrence Handle3741878
KH Süss C Arkona R Manteuffel K Adler (1993) ArticleTitleCalvin cycle multienzyme complexes are bound to chloroplast thylakoid membranes of higher plants in situ Proc Nat Acad Sci 90 5514–5518 Occurrence Handle11607406
DR Trentham CH McMurray CI Pogson (1969) ArticleTitleThe active chemical state of D-glyceraldehyde-3-phosphate dehydrogenase in its reactions with D-glyceraldehye-3-phosphate dehydrogenase, aldolase and triose phosphate isomerase Biochem J 114 19–24 Occurrence Handle4309306
SE Unkles JM Logsdon SuffixJr. K Robison JR Kinghorn JM Duncan (1997) ArticleTitleThe tigA gene is a transcriptional fusion of glycolytic genes encoding triose-phosphate isomerase and glyceraldehyde-3-phosphate dehydrogenase in oomycota J␣Bacteriol 179 6816–6823 Occurrence Handle9352934
Yañez AJ, Ludwig HC, Bertinat R, Spichiger C, Gatica R, Berlien G, Leon O, Brito M, Concha II, Slebe JC (2005) Different involvement for aldolase isoenzymes in kidney glucose metabolism: aldolase B but not aldolase A colocalizes and forms a complex with FBPase. J Cell Physiol 202: 743–753
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anderson, L.E., Gatla, N. & Carol, A.A. Enzyme co-localization in pea leaf chloroplasts: glyceraldehyde-3-P dehydrogenase, triose-P isomerase, aldolase and sedoheptulose bisphosphatase. Photosynth Res 83, 317–328 (2005). https://doi.org/10.1007/s11120-005-0790-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11120-005-0790-2