Abstract
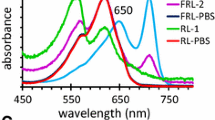
The marine picocyanobacterium Synechococcus sp. WH8102 was submitted to ultraviolet (UV-A and B) radiations and the effects of this stress on reaction center II and phycobilisome integrity were studied using a combination of biochemical, biophysical and molecular biology techniques. Under the UV conditions that were applied (4.3 W m−2 UV-A and 0.86 W m−2 UV-B), no significant cell mortality and little chlorophyll degradation occurred during the 5 h time course experiment. However, pulse amplitude modulated (PAM) fluorimetry analyses revealed a rapid photoinactivation of reaction centers II. Indeed, a dramatic decrease of the D1 protein amount was observed, despite a large and rapid increase in the expression level of the psbA gene pool. Our results suggest that D1 protein degradation was accompanied (or followed) by the disruption of the N-terminal domain of the anchor linker polypeptide LCM, which in turn led to the disconnection of the phycobilisome complex from the thylakoid membrane. Furthermore, time course analyses of in vivo fluorescence emission spectra suggested a partial dismantling of phycobilisome rods. This was confirmed by characterization of isolated antenna complexes by SDS-PAGE and immunoblotting analyses which allowed us to locate the disruption site of the rods near the phycoerythrin I—phycoerythrin II junction. In addition, genes encoding phycobilisome components, including α-subunits of all phycobiliproteins and phycoerythrin linker polypeptides were all down regulated in response to UV stress. Phycobilisome alteration could be the consequence of direct UV-induced photodamages and/or the result of a protease-mediated process.







Similar content being viewed by others
References
Araoz R, Hader DP (1999) Phycoerythrin synthesis is induced by solar UV-B in the cyanobacterium Nostoc. Plant Physiol Biochem 37:223–229
Araoz R, Hader DP (1997) Ultraviolet radiation induces both degradation and synthesis of phycobilisomes in Nostoc sp.: a spectroscopic and biochemical approach. FEMS Microbiol Ecol 23:301–313
Araoz R, Shelton M, Lebert M et al (1998) Differential behaviour of two cyanobacterium species to UV radiation. Artificial UV radiation induces phycoerythrin synthesis. J Photochem Photobiol B 44:175–183
Barbato R, Bergo E, Szabo I et al (2000) Ultraviolet B exposure of whole leaves of barley affects structure and functional organization of photosystem II. J Biol Chem 275:10976–10982
Booth MG, Jeffrey WH, Miller RV (2001) RecA expression in response to solar UVR in the marine bacterium Vibrio natriegens. Microb Ecol 42:531–539
Brahamsha B (1996) A genetic manipulation system for oceanic cyanobacteria of the genus Synechococcus. Appl Env Microbiol 62:1747–1751
Campbell D, Eriksson MJ, Oquist G et al (1998) The cyanobacterium Synechococcus resists UV-B by exchanging photosystem II reaction-center D1 proteins. Proc Natl Acad Sci USA 95:364–369
Capuano V, Braux AS, Tandeau de Marsac N et al (1991) The “anchor polypeptide” of cyanobacterial phycobilisomes. Molecular characterization of the Synechococcus sp. PCC 6301 apcE gene. J Biol Chem 266:7239–7247
Capuano V, Thomas JC, Tandeau de Marsac N et al (1993) An in vivo approach to define the role of the LCM, the key polypeptide of cyanobacterial phycobilisomes. J Biol Chem 268:8277–8283
Collier JL, Grossman AR (1994) A small polypeptide triggers complete degradation of light-harvesting phycobiliproteins in nutrient-deprived cyanobacteria. EMBO J 13:1039–1047
Dolganov N, Grossman AR (1999) A polypeptide with similarity to phycocyanin alpha-subunit phycocyanobilin lyase involved in degradation of phycobilisomes. J Bact 181:610–617
Ducret A, Sidler W, Wehrli E et al (1996) Isolation, characterization and electron microscopy analysis of a hemidiscoidal phycobilisome type from the cyanobacterium Anabaena sp. PCC 7120. Eur J Biochem 236:1010–1024
Eilers PH, Peters JCH (1988) A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol Model 42:199–215
Füglistaller P, Widmer H, Sidler W et al (1981) Isolation and characterisation of phycoerythrocyanin and chromatic adaptation of the thermophilic cyanobacterium Mastigocladus laminosus. Arch Microbiol 129:268–274
Fuller NJ, Marie D, Partensky F et al (2003) Clade-specific 16S ribosomal DNA oligonucleotides reveal the predominance of a single marine Synechococcus clade throughout a stratified water column in the Red Sea. Appl Env Microbiol 69:2430–2443
Genty B, Briantais J, Baker N (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:97–92
Gindt YM, Zhou J, Bryant DA et al (1994) Spectroscopic studies of phycobilisome subcore preparations lacking key core chromophores: assignment of excited state energies to the LCM, beta-18 and alpha AP-B chromophores. Biochim Biophys Acta 1186:153–162
Glauser M, Bryant DA, Frank G et al (1992) Phycobilisome structure in the cyanobacteria Mastigocladus laminosus and Anabaena sp. PCC 7120. Eur J Biochem 205:907–915
Glazer AN (1989) Light guides—Directional energy transfer in a photosynthetic antenna. J Biol Chem 264:1–4
Hader DP, Worrest RC, Kumar HD et al (1995) Effect of increase solar ultraviolet radiation on aquatic systems. Ambio 24:174–180
Hakala M, Tuominen I, Keranen M, Tyystjarvi T, Tyystjarvi E (2005) Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of Photosystem II. Biochim Biophys Acta 1706:68–80
He YY, Hader DP (2002) Involvement of reactive oxygen species in the UV-B damage to the cyanobacterium Anabaena sp. J Photochem Photobiol B Biol 66:73–80
He YY, Hader DP (2002) Reactive oxygen species and UV-B: effect on cyanobacteria. Photochem Photobiol Sci 1:729–736
He YY, Hader DP (2002) UV-B-induced formation of reactive oxygen species and oxidative damage of the cyanobacterium Anabaena sp.: protective effects of ascorbic acid and N-acetyl-L-cysteine. J Photochem Photobiol B Biol 66:115–124
He YY, Klisch M, Hader DP (2002) Adaptation of cyanobacteria to UV-B stress correlated with oxidative stress and oxidative damage. Photochem Photobiol 76:188–196
Herdman M, Castenholz RW, Waterbury JB et al (2001) Form-genus XIII. Synechococcus. In: Boone DR, Castenholz RW (eds) Bergey’s manual of systematic bacteriology, 2d edn, vol 1. Springer-Verlag, New York, pp 508–512
Hihara Y, Kamei A, Kanehisa M et al (2001) DNA microarray analysis of cyanobacterial gene expression during acclimation to high light. Plant Cell 13:793–806
Holzinger A, Lutz C (2006) Algae and UV irradiation: effects on ultrastructure and related metabolic functions. Micron 37:190–207
Huang LX, McCluskey MP, Ni H et al (2002) Global gene expression profiles of the cyanobacterium Synechocystis sp. strain PCC 6803 in response to irradiation with UV-B and white light. J Bact 184:6845–6858
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lao K, Glazer AN (1996) Ultraviolet-B photodestruction of a light-harvesting complex. Proc Natl Acad Sci USA 93:5258–5263
Llabres M, Agusti S (2006) Picophytoplankton cell death induced by UV radiation: evidence for oceanic Atlantic communities. Limnol Oceanogr 51:21–29
Marie D, Brussaard C, Partensky F et al (1999) Flow cytometric analysis of phytoplankton, bacteria and viruses. In: Robinson JP et al (eds) Current protocols in cytometry. International Society for Analytical Cytology, John Wiley and Sons, New York, pp 11.11.11–11.11.15
Mary I, Vaulot D (2003) Two-component systems in Prochlorococcus MED4: Genomic analysis and differential expression under stress. FEMS Microbiol Lett 226:135–144
Nishiyama Y, Allakhverdiev SI, Murata N (2006) A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta 1757:742–749
Ohnishi N, Allakhverdiev SI, Takahashi S et al (2005) Two-step mechanism of photodamage to photosystem II: step 1 occurs at the oxygen-evolving complex and step 2 occurs at the photochemical reaction center. Biochemistry 44:8494–8499
Ong LJ, Glazer AN (1987) R-phycocyanin II, a new phycocyanin occurring in marine Synechococcus species. J Biol Chem 262:6323–6327
Ong LJ, Glazer AN (1991) Phycoerythrins of marine unicellular cyanobacteria. I. Bilin types and locations and energy transfer pathways in Synechococcus spp. phycoerythrins. J Biol Chem 266:9515–9527
Palenik B, Brahamsha B, Larimer FW et al (2003) The genome of a motile marine Synechococcus. Nature 424:1037–1042
Pandey R, Chauhan S, Singhal GS (1997) UVB-induced photodamage to phycobilisomes of Synechococcus sp. PCC 7942. J Photochem Photobiol B 40:228–232
Partensky F, Hess WR, Vaulot D (1999) Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol Mol Biol Rev 63:106–127
Poppe F, Schmidt R, Hanelt D et al (2003) Effects of UV radiation on the ultrastructure of several red algae. Physiol Res 51:11–19
Rajagopal S, Murthy SDS (1996) Short term effect of ultraviolet-B radiation on photosystem 2 photochemistry in the cyanobacterium Synechococcus 6301. Biol Plant 38:129–132
Rajagopal S, Jha IB, Murthy SDS et al (1998) Ultraviolet-B effects on Spirulina platensis cells: modification of chromophore-protein interaction and energy transfer characteristics of phycobilisomes. Biochem Biophys Res Commun 249:172–177
Rinalducci S, Hideg E, Vass I et al (2006) Effect of moderate UV-B irradiation on Synechocystis PCC 6803 biliproteins. Biochem Biophys Res Commun 341:1105–1112
Rippka R et al (2000) Prochlorococcus marinus Chisholm et al. 1992 subsp. pastoris subsp. nov. strain PCC 9511, the first axenic chlorophyll a 2/b 2-containing cyanobacterium (Oxyphotobacteria). Int J Syst Evol Microbiol 50:1833–1847
Sah JF, Krishna KB, Srivastava H et al (1998) Effects of ultraviolet-B radiation on phycobilisomes of Synechococcus PCC 7942: Alterations in conformation and energy transfer characteristics. Biochem Mol Biol Int 44:245–257
Sicora C, Appleta SE, Brown CM et al (2006) Cyanobacterial psbA families in Anabaena and Synechocystis encode trace, constitutive and UVB-induced D1 isoforms. Biochim Biophys Acta 1757:47–56
Sidler WA (1994) Phycobilisome and phycobiliprotein structure. In: Bryant DA (ed) The molecular biology of cyanobacteria. Kluwer Academic Publishers, Netherlands, pp 139–216
Sinha RP, Klisch M, Groniger A et al (2001) Responses of aquatic algae and cyanobacteria to solar UV-B. Plant Ecol 154:219, 221–219, 236
Sinha RP, Lebert M, Kumar A et al (1995) Spectroscopic and biochemical analyses of UV effects on phycobiliproteins of Anabaena sp and Nostoc carmium. Botanica Acta 108:87–92
Six C, Thomas JC, Brahamsha B et al (2004) Photophysiology of the marine cyanobacterium Synechococcus sp. WH8102, a new model organism. Aquatic Microbial Ecol 35:17–29
Six C, Thomas J-C, Thion L et al (2005) Two novel phycoerythrin-associated linker proteins in the marine cyanobacterium Synechococcus sp. strain WH8102. J Bact 187:1685–1694
Six C, Worden AZ, Rodriguez F et al (2005) New insights into the nature and phylogeny of prasinophyte antenna proteins: Ostreococcus tauri, a case study. Mol Biol Evol 22:2217–2230
Smith RC et al (1992) Ozone depletion: ultraviolet radiation and phytoplankton biology in antarctic waters. Science 255:952–959
Strid A, Chow WS, Anderson JM (1994) UV-B damage and protection at the molecular level in plant. Photosynth Res 39:475–489
Vass I, Kirilovsky D, Etienne AL (1999) UV-B radiation-induced donor- and acceptor-side modifications of photosystem II in the cyanobacterium Synechocystis sp. PCC 6803. Biochem USA 38:12786–12794
Wendler J, John W, Scheer H et al (1986) Energy transfer in trimeric C-phycocyanin studied by picosecond fluorescence kinetics. Photochem Photobiol 44:79–85
Wilbanks SM, Glazer AN (1993) Rod structure of a phycoerythrin II-containing phycobilisome. II. Complete sequence and bilin attachment site of a phycoerythrin γ subunit. J Biol Chem 268:1236–1241
Wilson MI, Greenberg BM (1993) Specificity and photomorphogenic nature of ultraviolet-B-induced cotyledon curling in Brassica napus L. Plant Physiol 102:671–677
Xiong FS (2001) Evidence that UV-B tolerance of the photosynthetic apparatus in microalgae is related to the D1-turnover mediated repair cycle in vivo. J Plant Physiol 158:285–294
Xue L, Zhang Y, Zhang T et al (2005) Effects of enhanced ultraviolet-B radiation on algae and cyanobacteria. Crit Rev Microbiol 31:79–89
Yu MH, Glazer AN (1982) Cyanobacterial phycobilisomes. Role of the linker polypeptides in the assembly of phycocyanin. J Biol Chem 257:3429–3433
Yu MH, Glazer AN, Williams RC (1981) Cyanobacterial phycobilisomes. Phycocyanin assembly in the rod substructures of Anabaena variabilis phycobilisomes. J Biol Chem 256:13130–13136
Zsiros O, Allakhverdiev SI, Higashi S et al (2006) Very strong UV-A light temporally separates the photoinhibition of photosystem II into light-induced inactivation and repair. Biochim Biophys Acta 1757:123–129
Acknowledgements
This work was supported by the Region Bretagne (program IMPALA), the national program PROOF-UVECO, the EU programs MARGENES (QLRT-2001-01226) and SynChips, a flagship project of the European Network of Excellence Marine Genomics Europe. We warmly thank Jean-Claude Thomas (Ecole Normale Supérieure, Paris) for his kind contribution to the biochemical characterization of phycobilisome fractions and Douglas A. Campbell (Mount Allison University, NB Canada) for his useful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Six, C., Joubin, L., Partensky, F. et al. UV-induced phycobilisome dismantling in the marine picocyanobacterium Synechococcus sp. WH8102. Photosynth Res 92, 75–86 (2007). https://doi.org/10.1007/s11120-007-9170-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-007-9170-4