Abstract
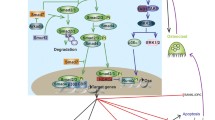
Wnts are a large family of growth factors that mediate fundamental biological processes like embryogenesis, organogenesis and tumorigenesis. These proteins bind to a membrane receptor complex comprised of a frizzled (FZD) G-protein-coupled receptor (GPCRs) and a low-density lipoprotein (LDL) receptor-related protein (LRP). The formation of this ligand-receptor complex initiates a number of intracellular signaling cascades that includes the canonical/β-catenin pathway, as well as several GPCR-mediated noncanonical pathways. In recent years, canonical Wnt signaling has been shown to play a substantial role in the control of bone formation. Clinical investigations have found that mutations in LRP-5 are associated with bone mineral density and fractures. For example, loss-of-function mutations in LRP-5 cause osteoporosis pseudoglioma syndrome, while gain-of-function mutations lead to high bone mass phenotypes. Studies of knockout and transgenic mouse models for Wnt pathway components like Wnt-10b, LRP-5/6, secreted frizzled-related protein-1, dickkopf-2, Axin-2 and β-catenin have demonstrated that canonical signaling modulates most aspects of osteoblast physiology including proliferation, differentiation, bone matrix formation/mineralization and apoptosis as well as coupling to osteoclastogenesis and bone resorption. Future studies in this rapidly growing area of research should focus on elucidating Wnt/FZD specificity in the control of bone cell function, the role of noncanonical pathways in skeletal remodeling, and direct effects of Wnts on cells of the osteoclast lineage.

Similar content being viewed by others
References
Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol 1998;14:59–88.
Miller JR. The Wnts. Genome Biology 2002; 3:REVIEWS3001.1–3001.15.
Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004;20:781–810.
Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet 2004;5:691–701.
Sharpe C, Lawrence N, Martinez Arias A. Wnt signalling: a theme with nuclear variations. Bioessays 2001;23:311–8.
Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet 2000;16:279–83.
Chen AE, Ginty DD, Fan C-M. Protein kinase A signaling via CREB control myogenesis induced by Wnt proteins. Nature 2005;433:317–22.
Pourquie O. A new canon. Nature 2005;433:208–9.
Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Genet Dev 2002;12:14–21.
Zorn AM. Wnt signaling: antagonistic dickkopfs. Curr Biol 2001;11:R592–5.
Jones SE, Jomary C. Secreted Frizzled-related proteins: searching for relationships and patterns. Bioessays 2002;24:811–20.
Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci 2003;116:2627–34.
Rawadi G, Roman-Roman S. Wnt signaling pathway: a new target for the treatment of osteoporosis. Expert Opin Ther Targets 2005;9:1063–77.
van Bezooijen RL, ten Dijke P, Papapoulos SE, Lowick CWGM. SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev 2005;16:319–27.
Bodine PV, Komm BS. Tissue culture models for studies of hormone and vitamin action in bone cells. Vitam Horm 2002;64:101–51.
Lian JB, Stein GS, Aubin JE. Bone formation: maturation and functional activities of osteoblast lineage cells. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. Washington, District of Columbia: American Society for Bone and Mineral Research; 2003. pp. 13–28.
Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene 2004;341:19–39.
Bodine PVN, Robinson JA, Bhat RA, Billiard J, Bex FJ, Komm BS. The role of Wnt signaling in bone and mineral metabolism. Clinical Reviews in Bone and Mineral Metabolism 2006;4:73–96.
Koay MA, Brown MA. Genetic disorders of the LRP5-Wnt signalling pathway affecting the skeleton. Trends Mol Med 2005;11:129–37.
Ferrari SL, Deutsch S, Antonarakis SE. Pathogenic mutations and polymorphisms in the lipoprotein receptor-related protein 5 reveal a new biological pathway for the control of bone mass. Curr Opin Lipidol 2005;16:207–14.
Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol 2002;157:303–14.
Kharode YP, Green PD, Marzolf JT, Zhao W, Askew R, Yaworsky P, et al. Alteration in bone density of mice due to heterozygous inactivation of LRP6. J Bone Miner Res 2003;18:S60.
Holmen SL, Giambernardi TA, Zylstra CR, Buckner-Berghuis BD, Resau JH, Hess JF, et al. Decreased BMD and limb deformities in mice carrying mutations in both LRP5 and LRP6. J Bone Miner Res 2004;19:2033–40.
Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, et al. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res 2003;18:960–74.
Akhter MP, Wells DJ, Short SJ, Cullen DM, Johnson ML, Haynatzki GR, et al. Bone biomechanical properties in LRP5 mutant mice. Bone 2004;35:162–9.
Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development 2005;132:49–60.
Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Developmental Cell 2005;8:727–38.
Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Developmental Cell 2005;8:739–50.
Holmen SL, Zylstra CR, Mukherjee A, Sigler RE, Faugere MC, Bouxsein ML, et al. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem 2005;280:21162–8.
Glass DA 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Developmental Cell 2005;8:751–64.
Jackson A, Vayssiere B, Garcia T, Newell W, Baron R, Roman-Roman S, et al. Gene array analysis of Wnt-regulated genes in C3H10T1/2 cells. Bone 2005;36:585–98.
Yu HM, Jerchow B, Sheu TJ, Liu B, Costantini F, Puzas JE, et al. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development 2005;132:1995–2005.
Li X, Liu P, Liu W, Maye P, Zhang J, Zhang Y, et al. Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet 2005;37:945–52.
Kang S, Bajnok L, Longo KA, Petersen RK, Hansen JB, Kristiansen K, et al. Effects of Wnt signaling on brown adipocyte differentiation and metabolism mediated by PGC-1alpha. Mol Cell Biol 2004;25:1272–82.
Longo KA, Wright WS, Kang S, Gerin I, Chiang SH, Lucas PC, et al. Wnt10b inhibits development of white and brown adipose tissues. J Biol Chem 2004;279:35503–9.
Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci USA 2005;102:3324–9.
Bodine PVN, Billiard J, Moran RA, Ponce-de-Leon H, McLarney S, Mangine A, et al. The Wnt antagonist secreted frizzled-related protein-1 controls osteoblast and osteocyte apoptosis. J Cell Biochem 2005;96:1212–30.
Bodine PV, Zhao W, Kharode YP, Bex FJ, Lambert AJ, Goad MB, et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol 2004;18:1222–37.
Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem 2005;280:41342–51.
Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem 2005;280:33132–40.
Lian JB, Trevant B, Symons J, Komm BS, Bodine PVN, Stein GS. The SFRP-1 gene, a Wnt antagonist, is expressed during embryonic development in bone and cartilage. J Bone Miner Res 2002;17:S405.
Trevant B, Symons J, Gaur T, Hussain S, Komm BS, Bodine PVN, et al. Normal embryonic mouse development does not require sFRP-1, a Wnt antagonist expressed in soft tissues and the skeleton. Submitted for publication, 2005.
Gaur T, Rich L, Lengner CJ, Hussain S, Trevant B, Ayers A, et al. Secreted frizzled-related protein-1 regulates Wnt Signaling for BMP2 induced chondrocyte differentiation. J Cell Physiol 2006;208:87–96.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bodine, P.V.N., Komm, B.S. Wnt signaling and osteoblastogenesis. Rev Endocr Metab Disord 7, 33–39 (2006). https://doi.org/10.1007/s11154-006-9002-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-006-9002-4