Abstract
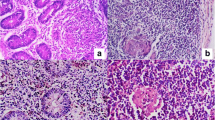
Comparative efficacy of faecal culture and IS900 Polymerase chain reaction (PCR) assay of faecal samples was investigated in 40 clinically suspected cases of Johne’s disease in dairy cattle. The sensitivity of faecal culture and PCR assay in this study was 52.5% (21/40) and 90% (36/40) respectively. All isolates appeared only on the mycobactin J supplemented Herrold’s egg yolk medium (HEYM) at 8–16 weeks post-inoculation, were acid-fast and were positive for IS900 PCR yielding a single amplicon of 217 bp. A total of 28 faecal samples out of 40 were positive by IS900 primary PCR assay for Mycobacterium avium subsp. paratuberculosis (Map) yielding an expected product of size 217 bp. Twelve faecal samples, which gave negative results in the primary PCR, were subjected to secondary PCR assay. Of the 12 samples, 8 gave positive results in the IS900 nested PCR (nPCR), which yielded a PCR product of 167 bp, proving better sensitivity of nPCR assay than single amplification PCR. PCR could detect additionally 15 samples as positive which were negative by faecal culture. The chi-square analysis showed a highly significant difference between the tests (P< 0.01). This study suggests that IS900-PCR-based detection of Map could be used as a potential diagnostic tool for rapid and effective Johne’s disease surveillance.
Similar content being viewed by others
Abbreviations
- PCR:
-
Polymerase chain reaction
- HEYM:
-
Herrold’s egg yolk medium
- Map:
-
Mycobacterium avium subsp. paratuberculosis
- OIE:
-
Office International des Epizooties
- nPCR:
-
Nested PCR
- HPC:
-
Hexadecylpyridinium chloride
- VAN:
-
Vancomycin, Amphotericin B and Nalidixic acid
- SDS:
-
Sodium dodecyl sulphate
- CTAB-NaCl:
-
Cetyl trimethyl ammonium bromide-Sodium chloride
References
Buergelt, C.D., Williams, E., Monif, G.R.G., Pinedo, P., Decker, J.H. 2006. Nested Polymerase chain reaction and prenatal detection of Mycobacterium avium subspecies paratuberculosis (Map) in bovine allantoic fluid and fetuses. International Journal of Applied Research in Veterinary Medicine, 4(3), 232–238.
Chiodini, R.J., Van Kruiningen, H.J., Merkal, R.S. 1984. Ruminant paratuberculosis (Johne’s disease): the current status and future prospects. Cornell Veterinarian, 74(3), 218–262.
Collins, D.M., Gabric, D.M., de Lisle, G.W. 1989. Identification of a repetitive DNA sequence specific to Mycobacterium paratuberculosis. FEMS Microbiology Letters, 51(1), 175–178. doi:10.1111/j.1574-6968.1989.tb03440.x
Collins, D.M., Hilbink, F., West, D.M., Hosie, B.D., Cooke, M.M., de Lisle, G.W. 1993a. Investigation of Mycobacterium paratuberculosis in sheep by faecal culture, DNA characterization and the polymerase chain reaction. Veterinary Record, 133(24), 599–600.
Collins, D.M., Stephens, D.M., de Lisle, G.W. 1993b. Comparison of polymerase chain reaction tests and faecal culture for detecting Mycobacterium paratuberculosis in bovine faeces. Veterinary Microbiology, 36(3–4), 289–299. doi:10.1016/0378-1135(93)90095-O
Cousins, D.V., Whittington, R., Marsh, I., Masters, A., Evans, R.J., Kluver, P. 1999. Mycobacteria distinct from Mycobacterium avium subsp. paratuberculosis isolated from the faeces of ruminants possess IS900-like sequences detectable by IS900 polymerase chain reaction: implications for diagnosis. Molecular and Cellular Probes, 13(6), 431–442. doi:10.1006/mcpr.1999.0275
Eishi, Y., Suga, M., Ishige, I., Kobayashi, D., Yamada, T., Takemura, T., Takizawa, T., Koike, M., Kudoh, S., Costabel, U., Guzman, J., Rizzato, G., Gambacorta, M., du Bois, R., Nicholson, A.G., Sharma, O.P., Ando, M. 2002. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. Journal of Clinical Microbiology, 40(1), 198–204. doi:10.1128/JCM.40.1.198-204.2002
Garrido, J.M., Cortabarria, N., Oguiza, J.A., Aduriz, G., Juste, R.A. 2000. Use of a PCR method on fecal samples for diagnosis of sheep paratuberculosis. Veterinary Microbiology, 77(3–4), 379–386. doi:10.1016/S0378-1135(00)00323-0
Giese, S.B., Ahrens, P. 2000. Detection of Mycobacterium avium subsp. paratuberculosis in milk from clinically affected cows by PCR and culture. Veterinary Microbiology, 77(3–4), 291–297. doi:10.1016/S0378-1135(00)00314-X
Halldorsdottir, S., Englund, S., Nilsen, S.F., Olsaker, I. 2002. Detection of Mycobacterium avium subsp. paratuberculosis by buoyant density centrifugation, sequence capture PCR and dot blot hybridisation. Veterinary Microbiology, 87(4), 327–340. doi:10.1016/S0378-1135(02)00082-2
Hasonova, L., Pavlik, I. 2006. Economic impact of paratuberculosis in dairy cattle herds: a review. Veterinarni Medicina, 51(5), 193–211.
Jenkins, P.A., Pattyn, S.R., Portaels, F. 1982. Diagnostic bacteriology. In: The biology of the mycobacteria. Ratledge, C. and Stanford, J. Academic press (London) Ltd., Vol. I, pp. 441–470.
Johnson, D.W., Muscoplat, C.C., Larsen, A.B., Thoen, C.O. 1977. Skin testing, faecal culture and lymphocyte immunostimulation in cattle inoculated with Mycobacterium paratuberculosis. American Journal of Veterinary Research, 38, 2023–2025.
Khare, S., Ficht, T.A., Santos, R.L., Romano, J., Ficht, A.R., Zhang, S., Grant, I.R., Libal, M., Hunter, D., Adams, L.G. 2004. Rapid and sensitive detection of Mycobacterium avium subsp. paratuberculosis in bovine milk and feces by a combination of immunomagnetic bead separation-conventional PCR and real-time PCR. Journal of Clinical Microbiology, 42(3), 1075–1081. doi:10.1128/JCM.42.3.1075-1081.2004
Mani, C., Selvakumar, N., Kumar, V., Narayanan, S., Narayanan, P.R. 2003. Comparison of DNA sequencing, PCR-SSCP and PhaB assays with indirect sensitivity testing for detection of rifampicin resistance in Mycobacterium tuberculosis. International Journal of Tuberculosis and Lung Disease, 7(7), 652–659.
Manning, E.J.B., Collins, M.T. 2001. Mycobacterium avium subsp. paratuberculosis: pathogen, pathogenesis and diagnosis. Review of Science and Technology in Office International des Epizooties, 20(1), 133–150.
Marsh, I.B., Whittington, R.J. 2001. Progress towards a rapid polymerase chain reaction diagnostic test for the identification of Mycobacterium avium subsp. paratuberculosis in faeces. Molecular and Cellular Probes, 15(2), 105–118. doi:10.1006/mcpr.2001.0345
Matthews, P.R., McDiarmid, A., Collins, P., Brown, A. 1978. The dependence of some strains of Mycobacterium avium on mycobactin for initial and subsequent growth. Journal of Medical Microbiology, 11(1), 53–57.
Merkal, R.S., Curran, B.J. 1974. Growth and metabolic characteristics of Mycobacterium paratuberculosis. Applied Microbiology, 28(2), 276–279.
Morrison, N.E., 1965. Circumvention of the mycobactin requirement of Mycobacterium paratuberculosis. Journal of Bacteriology, 89(3), 762–767.
Office International des Epizooties (OIE). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 2004. Paratuberculosis. Part 2., Section, 2.2., Chapter, 2.2.6
Ott, S.L., Wells, S.J., Wagner, B.A. 1999. Herd-level economic losses associated with Johne’s disease on US dairy operations. Preventive Veterinary Medicine, 40(3–4), 179–192. doi:10.1016/S0167-5877(99)00037-9
Pavlik, I., Matlova, L., Bartl, J., Svastova, P., Dvorska, L., Whitlock, R. 2000. Parallel faecal and organ Mycobacterium avium subsp. paratuberculosis culture of different productivity types of cattle. Veterinary Microbiology, 77(3–4), 309–324. doi:10.1016/S0378-1135(00)00316-3
Pinedo, P.J., Rae, D.O., Williams, J.E., Donovan, G.A., Melendez, P., Buergelt, C.D. 2008. Association among results of serum ELISA, faecal culture and nested PCR on milk, blood and faeces for the detection of paratuberculosis in dairy cows. Transboundary and Emerging Diseases, 55(2), 125–133. doi:10.1111/j.1865-1682.2007.01009.x
Rajeev, S., Zhang, Y., Sreevatsan, S., Motiwala, A.S., Byrum, B. 2005. Evaluation of multiple genomic targets for identification and confirmation of Mycobacterium avium subsp. paratuberculosis isolates using real-time PCR. Veterinary Microbiology, 105(3–4), 215–221. doi:10.1016/j.vetmic.2004.10.018
Reddacliff, L.A., Vadali, A., Whittington, R.J. 2003. The effect of decontamination protocols on the numbers of sheep strain of Mycobacterium avium subsp. paratuberculosis isolated from tissues and faeces. Veterinary Microbiology, 95(4), 271–282. doi:10.1016/S0378-1135(03)00181-0
Ronald, B.S.M. 2007. Development of Johne’s disease vaccine for sheep. Ph.D. thesis submitted to TANUVAS.
Schonenbrucher, H., Abdulmawjood, A., Failing, K., Bulte, M. 2008. New triplex real-time PCR assay for detection of Mycobacterium avium subsp. paratuberculosis in bovine feces. Applied and Environmental Microbiology, 74(9), 2751–2758. doi:10.1128/AEM.02534-07
Semret, M., Turenne, C.Y., Behr, M.A. 2006. Insertion sequence IS900 revisited. Journal of Clinical Microbiology, 44(3), 1081–1083. doi:10.1128/JCM.44.3.1081-1083.2006
Shin, S. 1989. Report of the committee on Johne’s disease. Proceedings of the 93rd Annual Meeting of the United States Animal Health Association, 93, 380–381.
Silverton, R.E., Anderson, M.J. 1961. Handbook of Medical Laboratory Formulae. Butterworths, London, pp. 118–119.
Singh, N., Vihan, V.S., Singh, S.V., Gupta, V.K. 1998. Prevalence of Johne’s disease in organized goat herds. Indian Journal of Animal Sciences, 68, 41–42.
Singh, S.V., Singh, A.V., Singh, R., Sandhu, K.S., Singh, P.K., Sohal, J.S., Gupta, V.K., Vihan, V.S. 2007. Evaluation of highly sensitive indigenous milk ELISA kit with faecal culture, milk culture and faecal-PCR for the diagnosis of bovine Johne’s disease (BJD) in India. Comparative Immunology, Microbiology and Infectious Diseases, 30, 175–186. doi:10.1016/j.cimid.2006.12.002
Sivakumar, P., Tripathi, B.N., Singh, N. 2005. Detection of Mycobacterium avium subsp. paratuberculosis in intestinal and lymph node tissues of water buffaloes (Bubalus bubalis) by PCR and bacterial culture. Veterinary Microbiology, 108(3–4), 263–270. doi:10.1016/j.vetmic.2005.04.002
Snedecor, G.W., Cochran, W.G. 1989. Statistical methods. 8th Edition, Iowa State University Press, Ames, Iowa.
Sockett, D.C., Carr, D.J., Collins, M.T. 1992. Evaluation of conventional and radiometric fecal culture and a commercial DNA probe for diagnosis of Mycobacterium paratuberculosis infections in cattle. Canadian Journal of Veterinary Research, 56(2), 148–153.
Sohal, J.S., Singh, S.V., Swati, S., Singh, A.V., Singh, P.K., Neelam, S., Komal, S., Narayanasamy, K., Maitra, A. 2007. Mycobacterium avium subspecies paratuberculosis diagnosis and strain typing- Present status and future developments. Indian Journal of Experimental Biology, 45(10), 843–852.
Stabel, J.R. 1997. An improved method for cultivation of Mycobacterium paratuberculosis from bovine fecal samples and comparison to three other methods. Journal of Veterinary Diagnosis and Investigation, 9, 375–380.
Stabel, J.R., Whitlock, R.H. 2001. An evaluation of a modified interferon– γ assay for the detection of paratuberculosis in dairy herds. Veterinary Immunology and Immunopathology, 79(1–2), 69–81. doi:10.1016/S0165-2427(01)00253-7
Sweeney, R.W., Whitlock, R.H., Rosenberger, A.E. 1992. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. Journal of Clinical Microbiology, 30(1), 166–171.
Tripathi, B.N., Munjal, S.K., Paliwal, O.P. 2002. An overview of paratuberculosis in animals. Indian Journal of Veterinary Pathology, 26, 1–10.
Tripathi, B.N., Sivakumar, P., Paliwal, O.P., Singh, N. 2006. Comparison of IS900 tissue PCR, bacterial culture, johnin and serological tests for diagnosis of naturally occurring paratuberculosis in goats. Veterinary Microbiology, 116(1–3), 129–137. doi:10.1016/j.vetmic.2006.03.017
Vinodh Kumar, O.R. 2003. Comparison of single intradermal test and polymerase chain reaction for the diagnosis of bovine paratuberculosis in organized farms. M.V.Sc. thesis submitted to TANUVAS.
Visser, I. 1999. Reproducibility of a faecal culture method for Mycobacterium paratuberculosis. Proceedings of the 6th International Colloquium on Paratuberculosis, Australia, pp. 41.
Whittington, R.J., Marsh, I., Choy, E., Cousins, D. 1998. Polymorphisms in IS1311, an insertion sequence common to Mycobacterium avium and M. avium subsp. paratuberculosis, can be used to distinguish between and within these species. Molecular and Cellular Probes, 12(6), 349–358. doi:10.1006/mcpr.1998.0194
Whittington, R.J., Sergeant, E.S. 2001. Progress towards understanding the spread, detection and control of Mycobacterium avium subsp. paratuberculosis in animal populations. Australian Veterinary Journal, 79(4), 267–278. doi:10.1111/j.1751-0813.2001.tb11980.x
Whittington, R.J. 2009. Factors affecting isolation and identification of Mycobacterium avium subsp. paratuberculosis from fecal and tissue samples in a liquid culture system. Journal of Clinical Microbiology, 47(3), 614–622. doi:10.1128/JCM.01986-08
Yayo Ayele, W., Fischer, O., Svastova, P., Alexa, M., Machackova, M., Pavlik, I. 2002. Dairy and beef cattle paratuberculosis survey in intensive and extensive farming conditions. Proceedings of the 7th International Colloquium on Paratuberculosis, Spain, pp. 340–344.
Acknowledgements
The authors thank The Dean, Rajiv Gandhi College of Veterinary and Animal Sciences, Puducherry, for providing facilities to carry out the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soumya, M.P., Pillai, R.M., Antony, P.X. et al. Comparison of faecal culture and IS900 PCR assay for the detection of Mycobacterium avium subsp. paratuberculosis in bovine faecal samples. Vet Res Commun 33, 781–791 (2009). https://doi.org/10.1007/s11259-009-9226-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11259-009-9226-3