Abstract
Purpose
This is the first clinical evaluation of a novel fluorescent imaging agent (Omocianine) for breast cancer detection with diffuse optical tomography (DOT).
Procedures
Eleven women suspected of breast cancer were imaged with DOT at multiple time points (up to 24 h) after receiving an intravenous injection of Omocianine (doses 0.01 to 0.1 mg/kg bodyweight). Breast MRI was obtained for comparison.
Results
Histopathology showed invasive cancer in ten patients and fibroadenoma in one patient. With the lowest dose of Omocianine, two of three lesions were detected; with the second dose, three of three lesions were detected; with the two highest doses, none of five lesions were detected. Lesion location on DOT showed excellent agreement with MRI. Optimal lesion-to-background signals were obtained after 8 h. No adverse events occurred.
Conclusions
Lowest doses of Omocianine performed best in lesion detection; DOT using a low-dose fluorescent agent is feasible and safe for breast cancer visualization in patients.
Similar content being viewed by others
Introduction
Optical breast imaging is a novel imaging technique that uses near-infrared (NIR) light to assess optical properties of tissue and is expected to play an important role in breast cancer detection. It dates back to 1929 when Cutler [1] investigated the shadows of light transmitted through the breast. Although large, highly vascular, malignant lesions could be detected, the method did not achieve sufficient sensitivity and specificity to be used in clinical practice at the time. During the last decade, progress in source and detector technology, light propagation modeling, and potential fluorescent contrast agents has resulted in a renewed interest in optical imaging [2]. Optical breast imaging uses NIR light in the wavelength range of 650 to 1,000 nm to assess optical properties of tissue. Functional information of tissue components, i.e., absorption characteristics of oxy- and deoxyhemoglobin, water, and lipid, can be obtained by combining images acquired at various wavelengths. Until now, studies have focused on using the intrinsic optical properties of the breast to visualize lesions without the use of fluorescent contrast agents. These studies described higher absorption for carcinomas than for the surrounding parenchyma due to increased blood content associated with angiogenesis [3–11]. However, intrinsic contrast alone is probably not sensitive enough for (early) lesion detection [12]. Optical breast imaging using a fluorescent contrast agent may improve lesion contrast and can potentially detect changes in breast tissue earlier. When fluorescent probes are excited by NIR light, they emit photons at predefined wavelength ranges, detectable by an optical imaging system. The fluorescent probes can either bind specifically to certain targets associated with cancer or can non-specifically accumulate at the tumor site, mostly by extravasation through leaky vessels. Several of these probes have already been tested successfully in animal experiments [13–15]. Major advantages of optical imaging with fluorescent probes are that it does not use any radioactive components (as in PET and SPECT) and that its sensitivity is very high (nanomolar to picomolar concentration range) compared to MRI. This technique has great potential in early breast cancer detection since in vivo imaging of molecular changes associated with breast cancer formation is technically feasible. Optical breast imaging provides us with functional information of the tissue, in contrast to X-ray mammography which merely relies on structural information. Additional advantages are that it uses no ionizing radiation, it is relatively inexpensive, which can result in repeated use even in young women, and is easily accessible. However, this technique is still in a very early phase of development. Systems need to be thoroughly evaluated first before the diagnostic value of the technique in a clinical setting can be determined. Few studies reported on optical breast imaging with the use of a fluorescent contrast agent. Two case reports described their experiences using the non-specific agent Indocyanine Green (ICG), the only fluorescent agent approved for use in humans today [16, 17]. Both groups observed a marked absorption increase in the malignant tumors due to accumulation of ICG, which, besides its fluorescent characteristics, also acts as a light absorber. In addition, differences in pharmacokinetics between malignant and benign lesions were found. However, ICG characteristics are suboptimal for fluorescence optical breast imaging; in particular, the low fluorescence quantum yield is a problem [18]. Nevertheless, in the study by Corlu et al. [19], it was shown that the use of ICG is feasible for fluorescence diffuse optical tomography (DOT). This was the first and thus far the only study to demonstrate fluorescence DOT in vivo in three women with breast cancer.
In our study, we investigated a non-specific cyanine-based fluorescent dye (Omocyanine) in patients using a DOT system dedicated for breast imaging. The aim of this study was to evaluate if our DOT system could detect malignant breast lesions using this new dye and to assess diagnostic efficacy, target dose, imaging window, and safety of the fluorescent contrast agent.
Methods
Patients
A total of 12 women (mean age 54, range 23–81) diagnosed with a 1- to 5-cm BI-RADS 4/5 breast lesion on X-ray mammography were prospectively included at the University Medical Center Utrecht, The Netherlands between January and July 2007.
Additional to the normal clinical diagnostic procedure of mammography and ultrasound, patients underwent optical imaging at different time points and received a single intravenous administration of the fluorescent contrast agent. MRI was obtained in all patients for comparison.
For every patient, the study consisted of a screening period to verify inclusion criteria, an imaging period during which optical images were acquired and the study drug was administered, and a follow-up period of 1 week to monitor adverse events.
Patients were excluded from the study if they were clinically unstable, had severe renal or hepatic problems, had a history of anaphylactic reactions, were pregnant or lactating, had more than two lesions in the ipsilateral breast, or if they underwent a breast biopsy less than 6 months before the study.
The study protocol was approved by the ethics committee of the University Medical Center Utrecht, The Netherlands, and written informed consent was obtained from all patients.
Contrast Material and Administration
As study drug, a single intravenous injection of Omocyanine (Bayer Schering Pharma, Berlin, Germany) suspended in saline (1 mg/mL) was used. Doses of 0.01, 0.02, 0.05, and 0.1 mg/kg body weight were administered in a dose escalating scheme with three patients per dose level, starting at the lowest dose. All doses were administered intravenously at a rate of 1 mL/s by manual injection, followed by a saline flush of 10–20 mL.
Before the injection of the study drug, baseline optical images were acquired for both breasts. After injection, optical images were acquired up to 24 h, with, respectively, eight and five imaging time points for the ipsilateral and contralateral breast (see flowchart in Fig. 1).
Safety Evaluations
Standard safety procedures (physical examinations, ECG recordings, laboratory tests) were carried out up to 24 h after study drug administration. Adverse events were recorded starting at the moment of study drug administration and throughout the 1-week follow-up period. Adverse events were defined as untoward medical occurrences which are not necessarily causally related to the drug administration. Signs and symptoms present before the study drug administration were recorded as baseline findings.
Optical Imaging
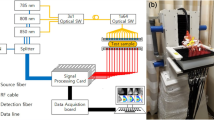
Data Acquisition
DOT scans were performed on a Philips Diffuse Optical Tomography system (Philips Healthcare, Best, The Netherlands). A patient was placed in the prone position on the system bed with one breast suspended in the cup [20]. The scanning module of the DOT system consists of a cup with a total of 507 optical fibers mounted on the surface. The 253 source fibers on all sides of the cup are connected to a fiber switch to direct the light of four continuous wave solid-state lasers into the cup. These fibers are interleaved with 254 detector fibers connected to 254 detectors. For each scan, the cup was filled with a matching fluid that has optical properties approximately equal to those of the average breast. This matching fluid enables a stable optical coupling between the fibers and the breast, as well as eliminating optical shortcuts of the diffuse light around the breast. During imaging, the breast was sequentially illuminated with continuous-wave near-infrared light from all source positions. Light emanating from the breast was detected for each source position by the detector fibers on all sides of the cup. Images were obtained at four discrete wavelengths (690, 730, 780, and 850 nm). Each breast is scanned separately. During one scan, the system was operated in two modes: transmission and fluorescence modes. The transmission measurements aimed at getting information on the optical absorption and scattering properties of the breast at four wavelengths. The fluorescent measurements were performed at one wavelength (730 nm) and the fluorescent signal was detected at a different wavelength (>750 nm), while the laser light was blocked by filters in the detection path. These measurements aimed at getting information on the concentration and distribution of the fluorescent dye in the breast.
The duration of the examination was approximately 1 min per wavelength and 5 min for the fluorescence, for a total of 9 min per breast.
Image Reconstruction
After optical data acquisition, three-dimensional absorption images were reconstructed by a linear reconstruction algorithm based on the Rytov approximation [21]. For reconstruction of three-dimensional fluorescence images, an algorithm based on the Born approximation was used [22]. One absorption image was calculated for each wavelength (four per scan). Since the reconstruction algorithm assumes constant scattering throughout the measurement cup, there is an influence of scattering variations on the reconstructed absorption images. If the average scattering of the breast is different from the scattering of the fluid, there will be an offset on the reconstructed breast absorption. If there is a variation of the scattering within the breast, the reconstructed image also shows features due to these variations. The fluorescence images (one per scan) only show fluorescence signal coming from the injected contrast agent; no absorption information is displayed in these images.
Magnetic Resonance Imaging
Contrast-enhanced breast MRI was performed on a 3.0-T clinical MR system (3.0 T Achieva, Philips Healthcare). Patients were placed in prone position on a dedicated four-element SENSE compatible phased-array bilateral breast coil (MRI devices, Würzburg, Germany) that was utilized for simultaneous imaging of both breasts. The scan protocol included an axial high-resolution T1-weighted fast gradient echo (HR-T1FFE) fat-suppressed series (TE/TR 1.7/4.5 ms; inversion delay SPAIR 130 ms; flip angle 10°; FOV 340 × 340 mm2, acquired voxel size 0.66 × 0.66 × 1.6 mm3, reconstructed voxel size 0.66 × 0.66 × 0.80 mm3), followed by an axial T2-weighted fat suppressed series (TE/TR 120/9,022 ms; inversion delay SPAIR 125 ms; flip angle 90°; FOV 340 × 340 mm2, acquired voxel size 1.01 × 1.31 × 2.0 mm3, reconstructed voxel size 0.66 × 0.66 × 2.00 mm3). Finally, dynamic contrast-enhanced fat-suppressed T1-weighted images (TE/TR 1.3/3.4 ms; flip angle 10°; FOV 320 × 320 mm, acquired voxel size 0.91 × 0.91 mm × 2.00 mm, reconstructed voxel size 0.83 × 0.83 mm × 1.00 mm; dynamic scan duration 60 s) were acquired, starting after intravenous bolus injection of 0.1 mmol/kg gadolinium (Magnevist, Bayer Schering Pharma).
Image Interpretation
MRI data were used as a benchmark for the optical image interpretation because these provide three-dimensional anatomical information.
When an enhanced fluorescent signal was visible on the optical images, its location was always compared to the position on MRI to see if the optical signal matched with the lesion location as seen on MRI.
A region of interest (ROI) was drawn at a single slice of the lesion site location for all fluorescent images over time. Separate ROIs were drawn for each imaging time point, each time matching the location of the lesion with the MRI data. For comparison, a similar ROI was drawn for each time point at the mirror image lesion site location of the contralateral breast where no lesion was found. The visibility of the lesions on DOT was assessed quantitatively by the DOT system. For all the ROIs that were drawn, quantitative values were computed from the images of the optical absorption coefficient obtained from the DOT system. The mean fluorescence intensity of the ROIs was determined. To calculate the lesion-to-background ratio, this value was divided by the mean fluorescence of the background, which included the rest of the breast on that slice except for the lesion and the nipple (very high intensity). The same was done for the mirror image ROI to compare the values in the ipsilateral and contralateral breast. Absorption images obtained before contrast administration were also assessed for lesion visibility; lesion-to-background ratios were calculated in the same way as for the fluorescence images in the cases that an enhanced fluorescent signal was observed.
To assess the pharmacokinetics of the new contrast agent in the breast, the uptake over time was compared quantitatively on the fluorescence images for the different ROIs in the ipsilateral and contralateral breast, and the optimal imaging time point was estimated.
The reference standard for final diagnosis of the lesions was histopathology after surgery or large core needle biopsy.
Statistics
To assess whether lesion size and administered dose were different between the lesions detected with DOT and the lesions not detected with DOT, a Mann–Whitney test was performed. The software package SPSS version 15.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical computations.
Results
Twelve women suspected of breast cancer were included in this study. One patient was excluded after screening measurements due to severe renal failure and therefore did not receive the contrast agent or the optical measurements. Eleven patients (mean age 54, range 23–81) underwent the total study procedures. Of these patients, histopathology results of the breast lesions showed invasive ductal carcinoma in eight patients (median lesion diameter 21 mm), invasive lobular carcinoma in two patients (median lesion diameter 40 mm), and a benign fibroadenoma (lesion diameter 15 mm) in one patient (Table 1).
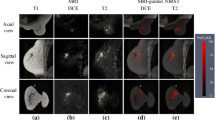
In the lowest dose group, lesions were detected in two patients on the fluorescence DOT images. In the second dose group, all three lesions were detected by DOT. No lesions were detected in the two highest dose groups. In total, five of the ten malignant lesions (50%) were visualized by DOT using the fluorescent contrast agent. The locations of lesions detected with DOT showed excellent agreement with MRI. Lesion location on the optical images was reproducible over time (Fig. 2). Optimal lesion-to-background signals were obtained after 8 h, ranging from 1.8 to 2.8 for the detected lesions. Non-specific fluorescent enhancement of glandular tissue was clearly visible on all optical fluorescence images, starting after 30 min and still evident 24 h later (Fig. 2). Contrast agent signal evidently increased with dose. Higher concentrations were problematic for the reconstruction algorithm due to the absorption of the agent being no longer negligible compared to the tissue absorption, an important assumption of the algorithm. A trend in the direction of a lower administered contrast agent dose was found for the detected lesions (exact p value 0.052). Median diameter was larger for the detected lesions (29 mm) than for the non-detected lesions (14.5 mm), but this difference was not significant (exact p value 0.13).
Example of the fluorescent images in a patient with invasive lobular carcinoma in the left breast (diameter 2.5 cm), optical images obtained 1, 2, 4, 8, and 24 h after fluorescent contrast agent injection. Contralateral and ipsilateral breast are shown, the lesion is indicated by the arrows, nipple is encircled, some reconstruction artifacts are visible in the top middle of the images. MRI is shown for comparison. The grayscale of the optical images is adjusted individually for each image to range from zero to five times the average background value. In this way, the varying contrast between lesion and background in the ipsilateral breast is visible.
The absorption images obtained with DOT before the contrast administration showed higher attenuation in the lesions than in the surrounding normal parenchyma. Mean lesion-to-background ratios were 1.8, 2.6, 1.6, and 1.4 for the wavelengths 690, 730, 780, and 850 nm, respectively.
Safety
No adverse events related to the study drug were observed during this study.
Discussion
The current study shows that DOT using a low dose of the fluorescent agent Omocyanine is feasible and safe for breast cancer visualization in patients. To our knowledge, this is the first report of the use of a novel fluorescent contrast agent for optical imaging of the breast in women suspected of breast cancer.
The fluorescent signal emitted by the contrast agent after excitation by NIR light could be detected with our DOT system in all patients. Since four different dose levels of the fluorescent agent were administered (ranging from 0.01 to 0.1 mg/kg body weight), the absolute increase of fluorescence signal with higher doses of the contrast agent could be clearly observed. Corlu et al. [19] were the first to demonstrate fluorescence DOT in patients using ICG. Other investigators did not use the fluorescence, but the absorbance characteristics of ICG for their optical imaging technique [16, 17]. In contrast to Corlu et al., we did not only investigate the breast with the lesion but also the contralateral healthy breast to obtain more information on the specificity of the technique.
In the present study, five of ten malignant lesions could be detected by fluorescence DOT. Best lesion-to-background ratios (range 1.8–2.8) were obtained after 8 h. Limitations in lesion detection primarily resulted from the reconstruction algorithm and the cup geometry of the current DOT system. Lowest doses of Omocyanine performed best in lesion detection, while higher concentrations were problematic for the reconstruction algorithm. The reason was that at a higher dose level, the contrast agent absorption was no longer negligible compared to the tissue absorption, which is an important assumption of the algorithm. Even at later imaging time points (up to 24 h), this reconstruction problem persisted due to the slow washout of the contrast agent. In two patients, the lesions were located close to their chest walls and could not be measured by the DOT system. Most probably, during the optical scans, these lesions were physically located too far above the upper optical fibers in the cup to influence the light pathways. Advances in cup geometry are feasible and should be realized to improve visualization of these lesions.
The other important limitation in this study was that Omocyanine is a non-specific contrast agent. Ideally, this fluorescent probe would accumulate at the tumor site by extravasation through leaky vessels. However, since it does not specifically bind to a cancer-associated target, enhancement of other normal tissues is also possible. This non-specific enhancement pattern was observed when comparing the uptake of the contrast agent over time for the lesion and mirror images in different patients. Differences were noted between patients whose breasts contained mostly fatty tissue and patients whose breasts contained mostly glandular (heterogeneously dense) tissue. To illustrate this observation, two examples are shown in Fig. 3. As shown in the graphs, uptake of the contrast agent was much lower in fatty tissue (patient 2) than in glandular tissue (patient 5) if the lesion is compared to the mirror image location. In the patients with a high content of glandular tissue in their breasts, the uptake at the mirror image site vs. the background was quite similar to the uptake at the lesion site vs. the background. Although the sample size is small and we should be cautious in generalizing these observations, it indicates that the distinction between glandular and malignant tissue could be problematic with this non-specific fluorescent contrast agent. Clearly, there is a need for target-specific fluorescent agents to be tested in patient studies using an optical imaging device.
Dye uptake over time for patient 2 and patient 5. The dye kinetics for patient 2 with mainly fatty breast tissue (BI-RADS density category 1) show lower uptake (reconstructed fluorescence signal) of the mirror image vs. the lesion, while for patient 5 with more glandular breast tissue (BI-RADS density category 3), the dye kinetics show similar uptake of the mirror image vs. the lesion.
In the absorption mode, DOT showed around twofold higher absorption at the site of the malignant lesions using wavelengths 690 and 730 nm, which is in agreement with previous studies and probably related to increased light absorption by the tumors’ higher hemoglobin content due to angiogenesis [7–9, 11, 19, 23].
To gain more insight in the composition of the breast, i.e., determine relative concentrations of (de)oxyhemoglobin, fat, and water, a spectral analysis which combines data of the different wavelengths in one model could be performed. Potentially, this spectral absorption information could improve the identification of malignant lesions and the specificity of DOT.
In conclusion, in this first feasibility study, we have shown that diffuse optical tomography with the use of the fluorescent contrast agent Omocyanine has the potential to safely visualize malignant breast tumors in patients. Development and especially the clinical translation of fluorescent probes with specific binding affinity for relevant molecular targets will be crucial to translate molecular breast imaging to clinical applications.
References
Cutler M (1929) Transillumination as an aid in the diagnosis of breast lesions. Surg Gynecol Obstet 48:721–728
Gibson AP, Hebden JC, Arridge SR (2005) Recent advances in diffuse optical imaging. Phys Med Biol 50(4):R1–43
Chance B, Nioka S, Zhang J et al (2005) Breast cancer detection based on incremental biochemical and physiological properties of breast cancers: a six-year, two-site study. Acad Radiol 12(8):925–933
Floery D, Helbich TH, Riedl CC et al (2005) Characterization of benign and malignant breast lesions with computed tomography laser mammography (CTLM): initial experience. Invest Radiol 40(6):328–335
Ntziachristos V, Yodh AG, Schnall MD, Chance B (2002) MRI-guided diffuse optical spectroscopy of malignant and benign breast lesions. Neoplasia 4(4):347–354
Rinneberg H, Grosenick D, Moesta KT et al (2005) Scanning time-domain optical mammography: detection and characterization of breast tumors in vivo. Technol Cancer Res Treat 4(5):483–496
Tromberg BJ, Cerussi A, Shah N et al (2005) Imaging in breast cancer: diffuse optics in breast cancer: detecting tumors in pre-menopausal women and monitoring neoadjuvant chemotherapy. Breast Cancer Res 7(6):279–285
Zhu Q, Cronin EB, Currier AA et al (2005) Benign versus malignant breast masses: optical differentiation with US-guided optical imaging reconstruction. Radiology 237(1):57–66
Intes X (2005) Time-domain optical mammography SoftScan: initial results. Acad Radiol 12(8):934–947
Taroni P, Torricelli A, Spinelli L et al (2005) Time-resolved optical mammography between 637 and 985 nm: clinical study on the detection and identification of breast lesions. Phys Med Biol 50(11):2469–2488
Cerussi A, Shah N, Hsiang D, Durkin A, Butler J, Tromberg BJ (2006) In vivo absorption, scattering, and physiologic properties of 58 malignant breast tumors determined by broadband diffuse optical spectroscopy. J Biomed Opt 11(4):044005
Leff DR, Warren OJ, Enfield LC et al (2007) Diffuse optical imaging of the healthy and diseased breast: a systematic review. Breast Cancer Res Treat 108(1):9–22
Hilger I, Leistner Y, Berndt A et al (2004) Near-infrared fluorescence imaging of HER-2 protein over-expression in tumour cells. Eur Radiol 14(6):1124–1129
Montet X, Ntziachristos V, Grimm J, Weissleder R (2005) Tomographic fluorescence mapping of tumor targets. Cancer Res 65(14):6330–6336
Bremer C, Ntziachristos V, Weitkamp B, Theilmeier G, Heindel W, Weissleder R (2005) Optical imaging of spontaneous breast tumors using protease sensing ‘smart’ optical probes. Invest Radiol 40(6):321–327
Intes X, Ripoll J, Chen Y, Nioka S, Yodh AG, Chance B (2003) In vivo continuous-wave optical breast imaging enhanced with Indocyanine Green. Med Phys 30(6):1039–1047
Ntziachristos V, Yodh AG, Schnall M, Chance B (2000) Concurrent MRI and diffuse optical tomography of breast after indocyanine green enhancement. Proc Natl Acad Sci U S A 97(6):2767–2772
Licha K, Riefke B, Ntziachristos V, Becker A, Chance B, Semmler W (2000) Hydrophilic cyanine dyes as contrast agents for near-infrared tumor imaging: synthesis, photophysical properties and spectroscopic in vivo characterization P. Photochem Photobiol 72:392–398
Corlu A, Choe R, Durduran T et al (2007) Three-dimensional in vivo fluorescence diffuse optical tomography of breast cancer in humans. Opt Express 15(11):6696–6716
Van de Ven SMWY, Elias SG, Wiethoff AJ et al (2009) Diffuse optical tomography of the breast: initial validation in benign cysts. Mol Imaging Biol 11:64–70. doi:10.1007/s11307-008-0176-x
Nielsen T, Brendel B, Ziegler R, Uhlemann F, Bontus C, Koehler T (2008) Linear image reconstruction for a diffuse optical mammography system in a non-compressed geometry using scattering fluid. Biomedical Optics 2008; OSA Technical Digest: BMD23
Scherleiter E, Zagar BG (2005) Optical tomography imaging based on higher order born approximation of diffuse photon densitiy waves. IEEE Trans Instrum Meas 54(4):1607–1611
Rice A, Quinn CM (2002) Angiogenesis, thrombospondin, and ductal carcinoma in situ of the breast. J Clin Pathol 55(8):569–574
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
van de Ven, S., Wiethoff, A., Nielsen, T. et al. A Novel Fluorescent Imaging Agent for Diffuse Optical Tomography of the Breast: First Clinical Experience in Patients. Mol Imaging Biol 12, 343–348 (2010). https://doi.org/10.1007/s11307-009-0269-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-009-0269-1