ABSTRACT
BACKGROUND
Framing of risk influences the perceptions of treatment benefit.
OBJECTIVE
To determine which risk framing format corresponds best to comprehensive multi-faceted information, and to compare framing bias in doctors and in patients.
DESIGN
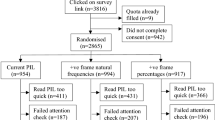
Randomized mail surveys.
PARTICIPANTS
One thousand four hundred and thirty-one doctors (56% response rate) and 1121 recently hospitalized patients (65% response rate).
INTERVENTION
Respondents were asked to interpret the results of a hypothetical clinical trial comparing an old and a new drug. They were randomly assigned to the following framing formats: absolute survival (new drug: 96% versus old drug: 94%), absolute mortality (4% versus 6%), relative mortality reduction (reduction by a third) or all three (fully informed condition). The new drug was reported to cause more side-effects.
MAIN MEASURE
Rating of the new drug as more effective than the old drug.
RESULTS
The proportions of doctors who rated the new drug as more effective varied by risk presentation format (abolute survival 51.8%, absolute mortality 68.3%, relative mortality reduction 93.8%, and fully informed condition 69.8%, p < 0.001). In patients these proportions were similar (abolute survival 51.7%, absolute mortality 66.8%, relative mortality reduction 89.3%, and fully informed condition 71.2%, p < 0.001). In both doctors (p = 0.72) and patients (p = 0.23) the fully informed condition was similar to the absolute risk format, but it differed significantly from the other conditions (all p < 0.01). None of the differences between doctors and patients were significant (all p > 0.1). In comparison to the fully informed condition, the odds ratio of greater perceived effectiveness was 0.45 for absolute survival (p < 0.001), 0.89 for absolute mortality (p = 0.29), and 4.40 for relative mortality reduction (p < 0.001).
CONCLUSIONS
Framing bias affects doctors and patients similarly. Describing clinical trial results as absolute risks is the least biased format, for both doctors and patients. Presenting several risk formats (on both absolute and relative scales) should be encouraged.
Similar content being viewed by others
REFERENCES
McGettigan P, Sly K, O’Connell D, Hill S, Henry D. The effects of information framing on the practices of physicians. J Gen Intern Med 1999;14:633–42
Edwards A, Elwyn G, Covey J, Matthews E, Pill R. Presenting risk information: a review of the effects of framing and other manipulations on patient outcomes. J Health Commun 2001;6:61–82
Moxey A, Dip G, O’Connell D, McGettigan P. Describing treatment effects to patients: How they are expressed makes a difference. J Gen Intern Med 2003;18:948–95
Covey J. A meta-analysis of the effects of presenting treatment benefits in different formats. Med Decis Making 2007;27:638–54
Malenka DJ, Baron JA, Johansen S, Wahrenberger JW, Ross JM. The framing effect of relative and absolute risk. J Gen Intern Med 1993;8:543–8
Hux JE, Naylor CD. Communicating the benefits of chronic preventive therapy: does the format of efficacy data determine patients’ acceptance of treatment? Med Decis Making 1995;15:152–7
Misselbrook D, Armstrong D. Patients’ responses to risk information about the benefits of treating hypertension. Br J Gen Practice 2001;51:276–9
Chao C, Studts JL, Abell T et al. Adjuvant chemotherapy for breast cancer : how presentation of recurrence risk influences decision-making. J Clin Oncol 2003;21:4299–305
Carling CLL, Kristoffersen DT, Montori VM et al. The effect of alternative summary statistics for communicating risk reduction on decisions about taking statins: a randomized trial. PLOS Med 2009;6:e1000134
Bucher HC, Weinbacher M, Gyr K. Influence of method of reporting study results on decision of physicians to prescribe drugs to lower cholesterol concentration. BMJ 1994;309:761–4
Epstein RM, Alper BS, Quill TE. Communicating evidence for participatory decision making. JAMA 2004;291:2359–66
Gigerenzer G, Wegwarth O, Feufel M. Misleading communication of risk. BMJ 2010;341:791–2
Schwartz PH, Meslin EM. The ethics of information: absolute risk reduction and patient understanding of screening. J Gen Intern Med 2008;23:867–70
Edwards A, Elwyn G. Understanding risk and lessons for clinical risk communication about treatment preferences. Qual Health Care 2001;10(Suppl 1):i9-i13
Stovring H, Gyrd-Hansen D, Kristiansen IS, Nexoe J, Nielsen JB. Communicating effectiveness of intervention for chronic diseases: what single format can replace comprehensive information? BMC Med Inform Decis Making 2008;8:25
Carling CLL, Kristoffersen DT, Oxman AD et al. The effect of how outcomes are framed on decisions about whether to take antihypertensive medication: a randomized trial. PLOS One 2010;5:e9469
Armstrong K, Schwartz JS, Fitzgerald G, Putt M, Ubel PA. Effect of framing as gain versus loss on understanding and hypothetical treatment choices: survival and mortality curves. Med Decis Making 2002;22:76–83
Peters E, Hart PS, Fraenkel L. Informing patients: the influence of numeracy, framing, and format of side effect information on risk perceptions. Med Decis Making. 2010; (epub).
Deom M, Agoritsas T, Perneger TV. What doctors think about the impact of managed care tools on quality of care, costs, autonomy, and relations with patients. BMC Health Services Res 2010;10:331
Agoritsas T, Courvoisier DS, Combescure C, Deom M, Perneger TV. Does prevalence matter to physicians in estimating post-test probability? A randomized trial. J Gen Intern Med 2011;26:373–8
Agoritsas T, Perneger TV. Patient-reported conformity of informed consent procedures and participation in clinical research. QJM Int J Med 2011;104:151–9.
Agoritsas T, Deom M, Perneger TV. Study design attributes influenced patients’ willingness to participate in clinical research: a randomized vignette-based study. J Clin Epidemiol. 2011;64–107.
Lee ET. Statistical Methods for Survival Data Analysis. 2nd edition. New York: John Wiley & Sons, 1992, p 133.
Young JM, Glasziou P, Ward JE. eneral practitioners’ self ratings of skills in evidence based medicine: validation study. BMJ. 2002;324:950–951.
Puhan MA, Steurer J, Bachmann LM, ter Riet G. A randomized trial of ways to describe test accuracy: the effect on physicians' post-test probability estimates. Ann Intern Med 2005;143:184–9.
Sox CM, Doctor JN, Koepsell TD, Christakis DA. The influence of types of decision support on physicians' decision making. Arch Dis Child 2009;94:185–90.
Schultz KF, Altman DG, Moher D. for the CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332.
Acknowledgment
The doctor survey was funded by an internal Research & Development grant, University Hospitals of Geneva; the patient survey was funded by the hospital’s Quality of Care Program. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Conflict of Interests
None disclosed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perneger, T.V., Agoritsas, T. Doctors and Patients’ Susceptibility to Framing Bias: A Randomized Trial. J GEN INTERN MED 26, 1411–1417 (2011). https://doi.org/10.1007/s11606-011-1810-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-011-1810-x