Abstract
Objective
Although a new matrix formulation fentanyl has been used throughout the world for cancer pain management, few data about its efficacy and clinical outcomes associated with its use in Chinese patients have been obtained. This study aimed to assess the efficacy and safety of the new system in Chinese patients with moderate to severe cancer pain.
Methods
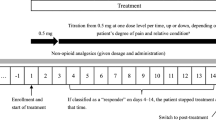
A total of 474 patients with moderate to severe cancer pain were enrolled in this study and were treated with the new transdermal fentanyl matrix patch (TDF) up to 2 weeks. All the patients were asked to record pain intensity, side effects, quality of life (QOL), adherence and global satisfaction. The initial dose of fentanyl was 25 μg/h titrated with opioid or according to National Comprehensive Cancer Network (NCCN) guidelines. Transdermal fentanyl was changed every three days.
Results
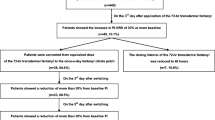
After 2 weeks. The mean pain intensity of the 459 evaluated patients decreased significantly from 5.63±1.26 to 2.03±1.46 (P<0.0001). The total remission rate was 91.29%, of which moderate remission rate 53.16%, obvious remission rate 25.49% and complete remission rate 12.64%. The rate of adverse events was 33.75%, 18.78% of which were moderate and 3.80% were severe. The most frequent adverse events were constipation and nausea. No fatal events were observed. The quality of life was remarkably improved after the treatment (P<0.0001).
Conclusion
The new TDF is effective and safe in treating patients with moderate to severe cancer pain, and can significantly improve the quality of life.
Similar content being viewed by others
References
Winslow M, Seymour J, Clark D. Stories of cancer pain: a historical perspective. J Pain Symptom Manage 2005; 29:22–31.
Portenoy RK. Opioid therapy for chronic nonmalignant pain: A review of the critical issues. J Pain Symptom Manage 1996; 11:203–217.
Hrachya Nersesyan, Konstantin V Slavin. Current approach to cancer pain management: Availability and implications of different treatment options. Ther Clin Risk Manag 2007; 3:381–400.
Pergolizzi J, Böger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract 2008; 8:287–313.
Schug SA, Merry AF, Acland RH. Treatment principles for the use of opioids in pain of nonmalignant origin. Drugs 1991; 42:228–239.
McQuay H. Opioids in pain management. Lancet 1999; 353:2229–2232.
Pereira J, Lawlor P, Vigano A, et al. Equianalgesic dose ratios for opioids: a critical review and proposals for long-term dosing. J Pain Symptom Manage 2001; 22:672–687.
Muijsers RB, Wagstaff AJ. Transdermal fentanyl: An updated review of its pharmacological properties and therapeutic efficacy in chronic cancer pain control. Drugs 2001; 61: 2289–2307.
Mystakidou K, Befon S, Kouskouni E, et al. From codeine to transdermal fentanyl for cancer pain control: a safety and efficacy clinical trial. Anticancer Res 2001; 21:2225–2230.
Murphy M, Carmichael AJ. Transdermal drug delivery systems and skin sensitivity reactions. Incidence and management. Am J Clin Dermatol 2000; 1:361–368.
Kongsgaard UE, Poulain P. Transdermal fentanyl for pain control in adults with chronic cancer pain. Eur J Pain 1998; 2:53–62.
Ahmedzai S, Brooks D. Transdermal fentanyl in cancer pain. The TTS-Fentanyl Multicentre Study Group. J Drug Dev 1994; 6:93–97.
Ahmedzai S, Brooks D. Transdermal fentanyl versus sustained-release oral morphine in cancer pain: preference, efficacy, and quality of life. The TTS-Fentanyl Comparative Trial Group. J Pain Symptom Manage 1997; 13:254–261.
Sloan PA, Moulin DE, Hays H. A clinical evalution of transdermal therapeutic system fentanyl for the treatment of cancer pain. J Pain Symptom Manage 1998; 16:102–111.
Grond S, Zech D, Lehmann KA, et al. Transdermal fentanyl in the long-term treatment of cancer pain: a prospective study of 50 patients with advanced cancer of the gastrointestinal tract or the head and neck region. Pain 1997; 69:191–198.
Payne R, Mathias SD, Pasta DJ, et al. Quality of life and cancer pain: satisfaction and side effects with transdermal fentanyl versus oral morphine. J Clin Oncol 1998;16:1588–1593.
Radbruch L, Sabatowski R, Petzke F, et al. Transdermal fentanyl for the management of cancer pain: a survey of 1005 patients. Palliat Med 2001; 15:309–321.
Kornick CA, Santiago-Palma J, Moryl N, et al. Benefit-risk assessment of transdermal fentanyl for the treatment of chronic pain. Drug Saf 2003; 26:951–973.
Sun Y, Gu WP. Guideline for cancer pain relief by three steps. 2nd ed. Beijing: Beijing Medical University Publishing House 2002; 100–101.
Gourlay GK. Treatment of cancer pain with transdermal fentanyl. Lancet Oncol 2001; 2:165–172.
Vielvoye-Kerkmeer AP, Mattern C, Uitendaal MP. Transdermal fentanyl in opioid-naive cancer pain patients: an open trial using transdermal fentanyl for the treatment of chronic cancer pain in opioid-naive patients and a group using codeine. J Pain Symptom Manage 2000; 19:185–192.
Donner B, Zenz M, Strumpf M, et al. Long-term treatment of cancer pain with transdermal fentanyl. J Pain Symptom Manage 1998; 15:168–175.
Jeal W, Benfield P. Transdermal fentanyl. A review of its pharmacological properties and therapeutic efficacy in pain control. Drugs 1997; 53:109–138.
Varvel JR, Shafer SL, Hwang SS, et al. Absorption characteristics of transdermally administered fentanyl. Anesthesiology 1989; 70:928–934.
Payne R, Chandler S, Einhaus M. Guidelines for the clinical use of transdermal fentanyl. Anti-Cancer Drugs 1995; 6:50–53.
Freynhagen R, von Giesen HJ, Busche P, et al. Switching from reservoir to matrix systems for the transdermal delivery of fentanyl: a prospective, multicenter pilot study in outpatients with chronic pain. J Pain Symptom management 2005; 30:289–297.
Marier JF, Lor M, Potvin D, et al. Pharmacokinetics, tolerability and performance of a novel matrix transdermal delivery system of fentanyl relative to the commercially available reservoir formulation in healthy subjects. J Clin Pharmacol 2006; 46:642–653.
Marier JF, Lor M, Morin J, et al. Comparative bioequivalence study between a novel matrix transdermal delivery system of fentanyl and a commercially available reservoir formulation. Br J Clin Pharmacol 2007; 63:121–124.
The TTS-Fentanyl Multicentre Study Group. Transdermal fentanyl in cancer pain. J Drug Dev 1994; 6:93–97.
Zech DF, Grond SU, Lynch J, et al. Transdermal fentanyl and initial dose-finding with patient-controlled analgesia in cancer pain. A pilot study with 20 terminally ill cancer patients. Pain 1992; 50:293–301.
Arner S, Meyerson BA. Lack of analgesic effect of opinoids on neuropathic and idiopathic forms of pain. Pain 1988; 33:11–23.
Kress HG, Von der Laage D, Hoerauf KH, et al. A randomized, open, parallel group, multicenter trial to investigate analgesic efficacy and safety of a new transdermal fentanyl patch compared to standard opioid treatment in cancer pain. J Pain and Symptom Management 2008; 36:268–279.
Collins JJ, Dunkel IJ, Gupta SK, et al. Transdermal fentanyl in children with cancer pain: feasibility, tolerability, and pharmacokinetic correlates. J Pediatr 1999; 134: 319–323.
Grond S, Radbruch L, Lehmann KA. Clinical pharmacokinetics of transdermal opioids: focus on transdermal fentanyl. Clin Pharmacokinet 2000; 38:59–89.
Sandler AN, Baxter AD, Katz J, et al. A double-blind, placebo-controlled trial of transdermal fentanyl after abdominal hysterectomy. Analgesic, respiratory, and pharmacokinetic effects. Anesthesiology 1994; 81: 1169–1180.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Yl., Song, Gh., Liu, Dq. et al. Multicenter clinical study for evaluation of efficacy and safety of transdermal fentanyl matrix patch in treatment of moderate to severe cancer pain in 474 Chinese cancer patients. Chin. J. Cancer Res. 23, 317–322 (2011). https://doi.org/10.1007/s11670-011-0317-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11670-011-0317-7