Abstract
Background
Recent physiological knowledge allows the design of bariatric procedures that aim at neuroendocrine changes instead of at restriction and malabsorption. Digestive adaptation is a surgical technique for obesity based in this rationale.
Methods
The technique includes a sleeve gastrectomy, an omentectomy and a jejunectomy that leaves initial jejunum and small bowel totaling at least 3 m (still within normal variation of adult human bowel length). Fasting ghrelin and resistin and fasting and postprandial GLP-1 and PYY were measured pre- and postoperatively.
Results
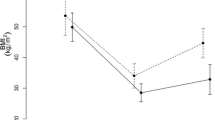
Patients: 228 patients with initial body mass index (BMI) varying from 35 to 51 kg/m2; follow-up: 1 to 5 years; average EBMIL% was 79.7% in the first year; 77.7% in the second year; 71.6% in the third year; 68.9% in the fourth year. Patients present early satiety and major improvement in presurgical comorbidities, especially diabetes. Fasting ghrelin and resistin were significantly reduced (P < 0.05); GLP-1 and PYY response to food ingestion was enhanced (P < 0.05). Surgical complications (4.4%) were resolved without sequela and without mortality. There was neither diarrhea nor detected malabsorption.
Conclusions
Based on physiological and supported by evolutionary data, this procedure creates a proportionally reduced gastrointestinal (GI) tract that amplifies postprandial neuroendocrine responses. It leaves basic GI functions unharmed. It reduces production of ghrelin and resistin and takes more nutrients to be absorbed distally enhancing GLP-1 and PYY secretion. Diabetes was improved significantly without duodenal exclusion. The patients do not present symptoms nor need nutritional support or drug medication because of the procedure, which is safe to perform.






Similar content being viewed by others
References
Drazen DL, Woods SC. Peripheral signals in the control of satiety and hunger. Curr Opin Clin Nutr Metabol Care 2003;6:621–9.
Ranganath LR, Beety JM, Morgan LM, et al. Attenuated GLP-1 secretion in obesity: cause or consequence? Gut 1996;38(6):916–9.
Batterham RL, Bloom SR. The gut hormone peptide YY regulates appetite. Ann NY Acad Sci 2003;994:162–8.
Santoro S, Velhote MCP, Malzoni CE, et al. Digestive adaptation: a new surgical proposal to treat obesity based in physiology and evolution. Einstein 2003;1(2):99–104.
Santoro S, Velhote MCP, Malzoni CE, et al. Preliminary results of digestive adaptation: a new surgical proposal to treat obesity based in physiology and evolution. Sao Paulo Med J 2006;124(4):192–7.
Santoro S, Malzoni CE, Velhote MCP, et al. Digestive adaptation with intestinal reserve: a neuroendocrine-based procedure for morbid obesity. Obes Surg 2006;16(10):1371–79.
Deitel M, Gawdat K, Melissas J. Reporting weight loss 2007. Obes Surg 2007;17:565–8.
Kenler HA, Brolin RE, Cody RP. Changes in eating behavior after horizontal gastroplasty and Roux-en-Y gastric bypass. Am J Clin Nutr 1990;52:87–92.
Le Roux CW, Aylwin SJB, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anoretic state, facilitate weight loss and improve metabolic parameters. Ann Surg 2006;243:108–14.
Stevens CE, Hume ID. Comparative physiology of the vertebrate digestive system. The mammalian gastrointestinal tract. Cambridge, United Kingdom: Cambridge University Press; 1995, p. 46–96.
Milton K. Primate diets and gut morphology: implications for human evolution. In: Harris M, Ross EB, editors. Food and Evolution. Toward a theory of human food habits. Philadelphia: Temple University Press; 1987.
Milton K. A hypothesis to explain the role of meat-eating in human evolution. Evol Anthropol 1999;8:11–21.
Aiello LC, Wheeler P. The expensive tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr Anthropol 1995;36:199–221.
Leonard WR, Robertson ML. Evolutionary perspectives on human nutrition: the influence of brain and body size on diet and metabolism. Am J Hum Biol 1994;6:77–88.
Lugari R, Dei Cas A, Ugolotti D, et al. Evidence for early impairment of glucagon-like peptide 1-induced insulin secretion in human type 2 (non insulin-dependent) diabetes. Horm Metab Res 2002;34(3):150–4.
Hounnou G, Destrieux C, Desme J, et al. Anatomical study of the length of the human intestine. Surg Radiol Anat 2002;24(5):290–4.
Muccioli G, Tschop M, Papotti M, et al. Neuroendocrine and peripheral activities of ghrelin: implications in metabolism and obesity. Eur J Pharmacol 2002;440(2–3):235–54.
Adkins RB Jr, Davies J. Gross and microscopic anatomy of the stomach and small intestine. In: Scott HW Jr, Sawyers JL, editors. Surgery of the stomach, duodenum and small bowel. Boston: Blackwell Scientific Publications; 1987. p. 45–60.
Tolessa T, Gutniak M, Holst JJ, et al. Inhibitory effect of glucagon-like peptide-1 on small bowel motility. Fasting but not fed motility inhibited via nitric oxide independently of insulin and somatostatin. J Clin Invest 1998;102:764–74.
Lin HC, Neevel C, Chen JH. Slowing intestinal transit by PYY depends on serotonergic and opioid pathways. Am J Physiol Gastrointest Liver Physiol 2004;286:G558–63.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292(14):1724–37.
Kreymann B, Williams G, Ghatei MA, et al. Glucagon-like peptide-1: a physiological incretin man. Lancet 1987;2(8571):1300–4.
Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood–brain barrier. J Mol Neurosci 2002;18:7–14.
Batterham RL, Bloom SR. The gut hormone peptide YY regulates appetite. Ann NY Acad Sci 2003;994:162–8.
van den Hoek AM, Heijboer AC, Corssmit EP, et al. PYY3-36 reinforces insulin action on glucose disposal in mice fed a high-fat diet. Diabetes 2004;53(8):1949–52.
Byrne TK. Complications of surgery for obesity. Surg Clin North Am 2001;81(5):1181–93.
Mason EE. Ileal transposition and enteroglucagon/GLP-1 in obesity (and diabetic?) surgery. Obes Surg 1999;9(3):223–8.
de Paula AL, Macedo AL, Prudente AS, et al. Laparoscopic sleeve gastrectomy with ileal interposition (“neuroendocrine brake”)—pilot study of a new operation. Surg Obes Relat Dis 2006;2(4):464–7.
Thulesen J. Glucagon-like peptide 2 (GLP-2), an intestinotrophic mediator. Current Protein and Peptide Science 2004;5(1):51–65.
Thulesen J, Hartmann B, Kissow H, et al. Intestinal growth adaptation and glucagon-like peptide 2 in rats with ileal-jejunal transposition or small bowel resection. Dig Dis Sci 2001;46(2):379–88.
Santoro S. Hipertrofia intestinal induzida por alimento e obesidade. Einstein 2005;3(4):310–2.
Santoro S, Velhote MCP, Malzoni CE, et al. Preliminary report: adaptive entero-omentectomy: physiological and evolutionary bases of an auxiliary treatment to type 2 diabetes. A report on the first two cases. Einstein 2004;2(3):193–98.
Milleo FQ, Malafaia O, Nassif PAN, et al. Comparative study of the effect of the Capella and Santoro Type II surgical techniques for treatment of obesity regarding BMI and peripheral triglyceridemia. Rev Bras Videocir 2006;4(4):151–61.
Meier JJ, Gethmann A, Götze O, et al. Glucagon-like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non-esterified fatty acids in humans. Diabetologia 2006;49:452–8.
Felber JP. Significance of the Randle-Mechanism in the etiology of diabetes type II. Horm Metab Res Suppl 1990;22:11–7.
Rahmouni K, Correia ML, Haynes WG, et al. Obesity-associated hypertension: new insights into mechanisms. Hypertension 2005;45(1):9–14.
Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Final report. Circulation 2002;106:3143–421.
Bergman RN, Van Citters GW, Mittelman SD, et al. Central role of the adipocyte in the metabolic syndrome. J Investig Med 2001;49(1):119–26.
McTernan CL, McTernan PG, Harte AL, Levick PL, Barnett AH, Kumar S. Resistin, central obesity, and type 2 diabetes. Lancet 2002;359(9300):46–7.
McTernan PG, McTernan CL, Chetty R, et al. Increased resistin gene and protein expression in human abdominal adipose tissue. J Clin Endocrinol Metab 2002;87(5):2407.
Hansen E, Hajri T, Abunmrad NN. Is all fat the same? The role of fat in the pathogenesis of metabolic syndrome and type 2 diabetes mellitus. Surgery 2006;139(6):711–6.
Acknowledgments
We deeply thank Mrs. Muriel Hallet and Mrs. Janice H. Hewins for the revision of the English version, Hospital Israelita Albert Einstein for the laboratory work (especially Dr. Morton Scheimberg and Dr. Cristóvão L.P. Mangueira), Dr Luís Fernado Lisboa for the statistical analyses, Ethicon Endosurgery, Inc. and Convidien Inc. for the donation of kits for entero-hormone dosages and some disposable materials used in the initial procedures of nonpaying patients.
Author information
Authors and Affiliations
Corresponding author
Additional information
Locations where the work was developed: Hospital Israelita Albert Einstein, Hospital da Policia Militar, São Paulo, and Hospital Vicentino, Ponta Grossa, Paraná, Brazil.
Rights and permissions
About this article
Cite this article
Santoro, S., Milleo, F.Q., Malzoni, C.E. et al. Enterohormonal Changes After Digestive Adaptation: Five-Year Results of a Surgical Proposal to Treat Obesity and Associated Diseases. OBES SURG 18, 17–26 (2008). https://doi.org/10.1007/s11695-007-9371-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-007-9371-0