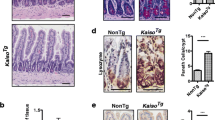
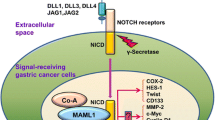
Abstract
The Notch receptor signaling pathway regulates expression of the basic helix-loop-helix transcription factor ATOH1 (Math1/Hath1) to determine cell fate in the intestine. In differentiating intestinal stem cells, high levels of Notch activity specify absorptive enterocyte/colonocyte differentiation, whereas high ATOH1 activity specifies secretory (goblet, enteroendocrine, and Paneth) cell differentiation. In colorectal cancer, ATOH1 is a tumor suppressor that is silenced in most tumors, while Notch is oncogenic and often highly active in human tumors. In other gastrointestinal malignancies with features of intestinal metaplasia, such as esophageal and gastric cancers, the Notch-ATOH1 pathway becomes activated. In cancers and preneoplastic tissues that retain the ability to activate ATOH1, therapeutic targeting of this pathway can be achieved by inhibiting Notch activity (with Notch-targeting antibodies or small-molecule inhibitors of γ-secretase). Thus, targeting the Notch-ATOH1 pathway represents a novel approach to differentiation therapy in gastrointestinal cancers.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–89.
Schroder N, Gossler A. Expression of Notch pathway components in fetal and adult mouse small intestine. Gene Expr Patterns. 2002;2(3–4):247–50.
Zecchini V, Domaschenz R, Winton D, Jones P. Notch signaling regulates the differentiation of post-mitotic intestinal epithelial cells. Genes Dev. 2005;19(14):1686–91.
Stanger BZ, Datar R, Murtaugh LC, Melton DA. Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci USA. 2005;102(35):12443–8.
• Riccio O, van Gijn ME, Bezdek AC, et al. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9(4):377–83. This article demonstrated that Notch1 and Notch2 are the key Notch receptors in the intestinal epithelium. It showed that the Notch target Hes1 directly represses p27 and p57 to control proliferation.
van Es JH, van Gijn ME, Riccio O, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435(7044):959–63.
Vooijs M, Ong CT, Hadland B, et al. Mapping the consequence of Notch1 proteolysis in vivo with NIP-CRE. Development. 2007;134(3):535–44.
Guilmeau S, Flandez M, Bancroft L, et al. Intestinal deletion of Pofut1 in the mouse inactivates notch signaling and causes enterocolitis. Gastroenterology. 2008;135(3):849–60. 60 e1-6.
Koo BK, Lim HS, Chang HJ, et al. Notch signaling promotes the generation of EphrinB1-positive intestinal epithelial cells. Gastroenterology. 2009;137(1):145–55. 55 e1-3.
Waterhouse CC, Johnson S, Phillipson M, et al. Secretory cell hyperplasia and defects in Notch activity in a mouse model of leukocyte adhesion deficiency type II. Gastroenterology. 2010;138(3):1079–90. e1-5.
Milano J, McKay J, Dagenais C, et al. Modulation of notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci. 2004;82(1):341–58.
Searfoss GH, Jordan WH, Calligaro DO, et al. Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional gamma-secretase inhibitor. J Biol Chem. 2003;278(46):46107–16.
Wong GT, Manfra D, Poulet FM, et al. Chronic treatment with the gamma-secretase inhibitor LY-411, 575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004;279(13):12876–82.
Ohlstein B, Spradling A. Multipotent drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315(5814):988–92.
Crosnier C, Vargesson N, Gschmeissner S, et al. Delta-notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development. 2005;132(5):1093–104.
Jensen J, Pedersen EE, Galante P, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24(1):36–44.
Suzuki K, Fukui H, Kayahara T, et al. Hes1-deficient mice show precocious differentiation of Paneth cells in the small intestine. Biochem Biophys Res Commun. 2005;328(1):348–52.
Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294(5549):2155–8.
Shroyer NF, Helmrath MA, Wang VY, et al. Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology. 2007;132(7):2478–88.
Vandussen KL, Samuelson LC. Mouse atonal homolog 1 directs intestinal progenitors to secretory cell rather than absorptive cell fate. Dev Biol. 2010
van Es JH, de Geest N, van de Born M, et al. Intestinal stem cells lacking the Math1 tumour suppressor are refractory to Notch inhibitors. Nat Commun. 2010;1(2):1–5.
Akiyama J, Okamoto R, Iwasaki M, et al. Delta-like 1 expression promotes goblet cell differentiation in Notch-inactivated human colonic epithelial cells. Biochem Biophys Res Commun. 2010;393(4):662–7.
• Kazanjian A, Noah T, Brown D, et al. Atonal Homolog 1 is required for growth and differentiation effects of Notch/gamma-secretase inhibitors on normal and cancerous intestinal epithelial cells. Gastroenterology. 2010. Established Atoh1 as the key target for GSI-mediated Notch effects on proliferation and differentiation of normal and cancerous intestinal epithelial cells.
Sato T, van Es JH, Snippert HJ, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2010
Guilmeau S, Flandez M, Mariadason JM, Augenlicht LH. Heterogeneity of Jagged1 expression in human and mouse intestinal tumors: implications for targeting Notch signaling. Oncogene. 2010;29(7):992–1002.
• Rodilla V, Villanueva A, Obrador-Hevia A, et al. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc Natl Acad Sci USA. 2009;106(15):6315–20. Provides evidence that β-catenin and Notch pathways have coordinate targets that mediate tumorigenesis. JAG1 is a key target for TCF4 effects and it is upregulated (along with NOTCH target/pathway genes) in human tumor samples; reduction of Jag1 inhibited tumor formation in mice.
Sander GR, Powell BC. Expression of notch receptors and ligands in the adult gut. J Histochem Cytochem. 2004;52(4):509–16.
Leow CC, Romero MS, Ross S, et al. Hath1, down-regulated in colon adenocarcinomas, inhibits proliferation and tumorigenesis of colon cancer cells. Cancer Res. 2004;64(17):6050–7.
• Tsuchiya K, Nakamura T, Okamoto R, et al. Reciprocal targeting of Hath1 and beta-catenin by Wnt glycogen synthase kinase 3beta in human colon cancer. Gastroenterology. 2007;132(1):208–20. This article demonstrates Wnt/β-catenin pathway regulation of Atoh1 protein stability by GSK3-beta phosphorylation and proteasomal degradation.
Peignon G, Durand A, Cacheux W, et al. Complex interplay between {beta}-catenin signalling and Notch effectors in intestinal tumorigenesis. Gut. 2011;60(2):166–76.
Yin L, Velazquez OC, Liu ZJ. Notch signaling: emerging molecular targets for cancer therapy. Biochem Pharmacol. 2010;80(5):690–701.
Reedijk M, Odorcic S, Zhang H, et al. Activation of Notch signaling in human colon adenocarcinoma. Int J Oncol. 2008;33(6):1223–9.
Sikandar SS, Pate KT, Anderson S, et al. NOTCH signaling is required for formation and self-renewal of tumor-initiating cells and for repression of secretory cell differentiation in colon cancer. Cancer Res. 2010;70(4):1469–78.
• Fre S, Pallavi SK, Huyghe M, et al. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc Natl Acad Sci USA. 2009;106(15):6309–14. Showed that NICD expression can enhance tumorigenesis in mice. Also showed a requirement of TCF4 for NICD-induced cell proliferation but not for inhibition of goblet cell differentiation.
Wood LD, Parsons DW, Jones S, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318(5853):1108–13.
Sancho R, Jandke A, Davis H, et al. F-box and WD repeat domain-containing 7 regulates intestinal cell lineage commitment and is a haploinsufficient tumor suppressor. Gastroenterology. 2010;139(3):929–41.
Ghaleb AM, Aggarwal G, Bialkowska AB, et al. Notch inhibits expression of the Kruppel-like factor 4 tumor suppressor in the intestinal epithelium. Mol Cancer Res. 2008;6(12):1920–7.
Akiyoshi T, Nakamura M, Yanai K, et al. Gamma-secretase inhibitors enhance taxane-induced mitotic arrest and apoptosis in colon cancer cells. Gastroenterology. 2008;134(1):131–44.
Aleksic T, Feller SM. Gamma-secretase inhibition combined with platinum compounds enhances cell death in a large subset of colorectal cancer cells. Cell Commun Signal. 2008;6:8.
Wu Y, Cain-Hom C, Choy L, et al. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464(7291):1052–7.
•• Bossuyt W, Kazanjian A, De Geest N, et al. Atonal homolog 1 is a tumor suppressor gene. PLoS Biol. 2009;7(2):e39. Established Atoh1 as a tumor suppressor and showed deletion and/or methylation of ATOH1 as the mechanism of silencing in primary colorectal cancers.
Park ET, Oh HK, Gum Jr JR, et al. HATH1 expression in mucinous cancers of the colorectum and related lesions. Clin Cancer Res. 2006;12(18):5403–10.
Heiskala K, Arola J, Heiskala M, Andersson LC. Expression of Reg IV and Hath1 in neuroendocrine neoplasms. Histol Histopathol. 2010;25(1):63–72.
Noah TK, Kazanjian A, Whitsett J, Shroyer NF. SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Exp Cell Res. 2010;316(3):452–65.
Yeh TS, Wu CW, Hsu KW, et al. The activated Notch1 signal pathway is associated with gastric cancer progression through cyclooxygenase-2. Cancer Res. 2009;69(12):5039–48.
Mutoh H, Sakamoto H, Hayakawa H, et al. The intestine-specific homeobox gene Cdx2 induces expression of the basic helix-loop-helix transcription factor Math1. Differentiation. 2006;74(6):313–21.
Sekine A, Akiyama Y, Yanagihara K, Yuasa Y. Hath1 up-regulates gastric mucin gene expression in gastric cells. Biochem Biophys Res Commun. 2006;344(4):1166–71.
Liu T, Zhang X, So CK, et al. Regulation of Cdx2 expression by promoter methylation, and effects of Cdx2 transfection on morphology and gene expression of human esophageal epithelial cells. Carcinogenesis. 2007;28(2):488–96.
Morrow DJ, Avissar NE, Toia L, et al. Pathogenesis of Barrett’s esophagus: bile acids inhibit the Notch signaling pathway with induction of CDX2 gene expression in human esophageal cells. Surgery. 2009;146(4):714–21. discussion 21–2.
•• Menke V, van Es JH, de Lau W, et al. Conversion of metaplastic Barrett’s epithelium into post-mitotic goblet cells by gamma-secretase inhibition. Dis Model Mech. 2010;3(1–2):104–10. In this paper, γ-secretase inhibitor treatment activates ATOH1 and converts intestinal metaplastic epithelial cells (Barrett’s esophagus) into non-proliferating, differentiated goblet cells.
Acknowledgments
Supported by NIH grants R01 CA142826 and R03 DK084167.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kazanjian, A., Shroyer, N.F. NOTCH Signaling and ATOH1 in Colorectal Cancers. Curr Colorectal Cancer Rep 7, 121–127 (2011). https://doi.org/10.1007/s11888-011-0090-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-011-0090-5