Abstract
A vaccine against recurrent vulvovaginal candidiasis (RVVC) would benefit a large number of women who suffer from this debilitating syndrome. To date, several antigen formulations have been tested with modest results. In this article, we review the latest vaccine study reported in the literature. The candidate is a β-glucan conjugate administered with a human compatible adjuvant. Results in a mouse model of vaginitis were again modest for protection. However, the study included live animal imaging to quantify fungal burden; animals were challenged with a Candida strain carrying a gene encoding a glycophosphatidylinositol (GPI)-linked cell wall protein and luciferase. Fungal burden was expressed as photons following substrate administration. Protection appeared to be mediated by β-glucan antibodies. Although modest protection was observed, the imaging system was less variable than semi-quantitative plate counts of vaginal lavage fluid. Despite these advances in evaluating protection, a vaccine candidate against RVVC worthy of clinical testing remains elusive.
Similar content being viewed by others
Introduction
Vulvovaginal candidiasis (VVC) and recurrent VVC (RVVC), caused by Candida species, is a major public health problem affecting a large number of otherwise healthy women of child-bearing age [1–4]. Although uncomplicated VVC, defined as single episodes with known disposing factors, usually responds to treatment, RVVC cases marked by idiopathic recurrent episodes can be virtually untreatable. Both forms of disease have a significant effect on quality of life and together pose a huge burden to the health care system, which are the driving forces for novel treatment and prevention strategies. To this end, immunotherapies or vaccines against this and other fungal diseases are under investigation [5].
The immune pathogenesis of VVC and RVVC has been intensely studied over the past two decades [6–22], but the factors associated with resistance and susceptibility have remained largely unknown until recently when reports surfaced revealing a major paradigm change. These studies showed that instead of susceptibility being associated with a local immune deficiency, symptomatic infection in susceptible women results from a response to Candida albicans by vaginal epithelial cells that promotes an aggressive migration of polymorphonuclear neutrophils (PMNs) into the vaginal canal. The ensuing acute inflammatory response ultimately causes the symptoms associated with vaginitis, but is non-clearing in that the Candida is unaffected by the inflammatory cells [23]. The vaginal epithelium of women resistant to VVC, on the other hand, does not provoke an inflammatory response upon contact with Candida, thus VVC-resistant women may harbor the fungus, but only in its commensal form. This information highlights the importance of epithelial cells and the innate immune system as being intimately involved in susceptibility or resistance to symptomatic condition. Indeed, it was recently reported through an established animal model of vaginal candidiasis that calcium-binding proteins, S100A8 and S100A9, produced by vaginal epithelial cells following interaction with Candida is the central trigger for the chemotaxis of PMNs into the vaginal cavity [24•]. Hence, the new hypothesis is that VVC is associated with signals following Candida-vaginal epithelial cell interactions that promote a nonprotective inflammatory PMN response and concomitant clinical symptoms. The amount of Candida present in the vagina is crucial to the epithelial cell-mediated signal(s) and this amount can be variable for different groups of women. In effect, the epithelial cells of women are either sensitive or insensitive to Candida and secrete “danger” signals when the threshold of resistance is breached. For example, women with RVVC inevitably acquire an infection shortly after completing a regimen of antifungal therapy. Vaginal epithelial cells in these women are extremely sensitive to Candida and signal the PMN infiltration after exposure to very low numbers of the fungus. Vaginal epithelial cells of women with an infrequent history of VVC due to known predisposing factors have a lower sensitivity for Candida and thus do not signal the PMNs until the population numbers of Candida increase following growth-promoting conditions (i.e., antibiotic therapy, hormone replacement therapy, use of high-estrogen oral contraceptives, pregnancy, or diabetes mellitus). Vaginal epithelial cells in women with no history of VVC are highly insensitive to Candida. Thus, although the population numbers of Candida can increase under similar conditions, they rarely, if ever, cross a threshold where the epithelial cells will stimulate PMN migration and are spared development of symptoms of vaginitis.
The lack of protection by adaptive responses to Candida in the vagina appears to involve strong immunoregulation, as exemplified in infected animals by the presence of TGF-β, γ/δ T cells, and plasmacytoid dendritic cells [20, 25, 26]. The immunoregulatory factors may well be the result of an evolution of immunity away from responses leading to strong inflammatory reactions to a commensal organism in the reproductive tract. The exception seems to be in the rat model of vaginal candidiasis, where both T and B cells have been implicated in protection against the infection [27, 28] that otherwise clears spontaneously, uncharacteristic of the clinical condition or mouse model. Candida-specific antibodies are detected clinically and can be induced by immunization and antigen administration in animal models [29–31]. However, little to no Candida-specific antibody has been detected from a vaginal inoculation in the mouse model [32]. Humoral immunity induced as a result of infection is generally not considered protective and may be under some form of immunoregulation as well. However, parenteral immunization with subcellular antigens enriched for Candida cell wall surface mannoproteins may induce protective antibody responses against experimental vaginal infection [33], and monoclonal antibodies against certain mannan components are protective when given intraperitoneally [33–35]. Although rapid complement opsonization of the fungal cells with antibody is involved in the mechanism of protection against hematogenously disseminated candidiasis [36], a mechanism by which antibodies protect against vaginal infection is unknown. This topic is further complicated by the finding that antibodies against Candida may be protective, unprotective, and indifferent. Protective antibodies include IgM and IgG3 antibodies in the mouse and IgG2b antibodies in the rat [30, 34, 35].
Based on this information, if a vaccine were to be effective against Candida vaginitis, it would most likely have to be antibody mediated and carefully characterized and confirmed as a “protective” antibody. The vaccine would function, by mechanisms yet to be defined, to reduce the number of Candida in susceptible women so that the epithelial cell danger signal does not get initiated and the acute inflammatory response causing the symptoms is ablated or avoided.
Over the past 15 years, a limited number of reports have been published on vaccination attempts against Candida vaginitis. Most included simple immunization schemes with antigen and adjuvant or vectors as a means to understand the host response rather than test primary vaccine candidates. Antigens included Hsp-90, Als3, β-glucan (laminarin), mannoprotein, secreted aspartyl proteases (SAPs), and yeast killer toxin anti-idiotypic antibodies [34, 37–39, 40••, 41, 42]. Adjuvants or carrier proteins included liposomes, cholera toxin, diphtheria toxoid, and alum. DNA preparations were also attempted. Routes included intravaginal, intradermal, intranasal, and intravenous. Most were performed in the rat model of vaginal candidiasis, which is quite distinct from the mouse model and does not parallel the clinical syndrome. Others were performed in the more clinically relevant mouse model. In any case, some protection was observed, the predominant response being antibody mediated.
Recent Vaccine Studies
During the past year, there was only one report testing a vaccine candidate against Candida vaginitis in a mouse model. The candidate is a β-glucan conjugate vaccine that induces anti–β-glucan antibodies; the investigators also reported on an imaging approach to detect the organism and analyze protection [43••]. The β-glucan vaccine (laminarin-CRM) (obtained from Novartis Vaccines and Diagnostics, Cambridge, MA, but based on earlier studies by this group [41]) was formulated with a human-compatible MF59 adjuvant and administered to mice subcutaneously, followed 14 and 21 days later by booster immunizations intraperitoneally of the vaccine without adjuvant. The mice were then placed into pseudoestrus 3 days later (day 24) and weekly thereafter. On days 30 and 31, the mice were challenged by intravaginal administration of 107 C. albicans CA1398 blastoconidia carrying the reported gene for the fungus, ACT1p-gLUC59 or, simply, gLUC59. This reporter system is based on expression of the C. albicans PGA59 gene that encodes a GPI-linked cell wall protein [44] along with Gaussia princeps luciferase [45], resulting in surface-expressed luciferase on the fungal cells. Following intravginal challenge, the fungal burden may be assessed by intravaginal administration of the luciferase substrate, coelenterazine, which results in photon emission and enables IVIS Imaging System 200 (Caliper Life Sciences, Hopkinton, MA) imaging of such mice under anesthesia. Total photon emission from the vaginal area was captured and quantified with Living Image software (Caliper Life Sciences). In some experiments, vaginal lavages were collected and fungal burden was determined by semi-quantitative plate counts, or vaginae were excised and processed for periodic acid-Schiff staining. Additional experimental designs included passive immunization with vaginal fluids from immunized mice or β-glucan antibodies. Vaginal lavage fluids were assayed for β-glucan antibodies and antibody class by enzyme-linked immunosorbent assay.
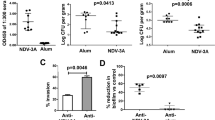
The first set of experiments was to confirm that the inoculating strain could be detected by the imaging system and if any protection was achieved. Accordingly, mice were immunized with the vaccine/adjuvant or adjuvant alone and challenged with the gLUC59 strain or the strain devoid of gLUC59. Over a 25-day period, photons were only detected in the mice challenged with the gLUC59 strain and protection was observed only in vaccinated mice beginning at day 11 post-challenge (Fig. 1). However, the protection was modest, with the greatest differences occurring as a result of seemingly uncharacteristic increases in photon emissions for one or more animals in the adjuvant-only control group compared to the relatively low photon emission by the remaining control animals and all the vaccinated animals. A comparison of results from photon emission and quantitative culture of lavage fluid indicated less variability in the photon analysis. Concurrent protection by both measures of fungal burden was observed only at day 13, even though correlation coefficients ranged from 0.86 to 0.99 on all days. Periodic acid-Schiff staining of tissue sections for day 17 post-challenge showed distinct differences between adjuvant and vaccine-adjuvant–treated mice, but the large differences noted did not match well with the modest differences obtained by imaging and colony-forming unit data.
In vivo imaging of vaccinated mice vaginally inoculated with Candida albicans cells expressing gLUC. Mice previously treated with adjuvant (MF59) or vaccinated with adjuvant plus Lam-CRM (M59 + LAM), under pseudoestrus conditions, were inoculated for 2 consecutive days with 10 μL of a 109 cell/mL suspension of C. albicans cell expressing gLUC into the vaginal lumen. After 4, 7, 11, and 13 days, post-inoculation mice were treated intravaginally with 10 μg of coelenterazine and imaged in the IVIS-200 imaging system under 2.5% isoflurane anesthesia. (From Pietrella et al. [43••], with permission from Elsevier.)
Anti–β-glucan antibody analyses of serum starting on day 31 showed a greater optical density reading due to IgG antibody in vaccine compared to adjuvant-treated mice. The differences between the two groups persisted through day 62 of the experiment, but antibody titering of the sera was not done at any time point. The same analysis of lavage fluid showed a single spike of IgG in the vaccine-treated mice at day 31 only. Mice given a passive administration of lavage fluid collected 42 days after the first immunization followed by a challenge with the Candida gLUC strain showed a small but significant transient protection (via photon analysis) through 5 days compared to lavage fluid from adjuvant-treated mice. Unfortunately, a critical control showing that the protective factor could be absorbed-out of the fluid by Candida cells was not included. Nonetheless, a similar modest transient protection, as indicated by photon imaging, was observed by passive immunization of an IgG2b monoclonal antibody (mAb2G8) specific for β-glucan and previously shown to be protective against candidiasis [41]. Taken together, these results suggest, but do not prove, that protection observed from the lavage fluid was due to anti–β-glucan antibodies in the lavage fluid.
Overall, the authors provide evidence that the imaging system has merits in assessing protection in the mouse model of vaginal candidiasis. The limitation, of course, is the need for a luciferase-expressing recombinant fungal strain. Importantly, they also provide evidence for protection induced by a β-glucan conjugate vaccine. This protection may be mediated by antibody, but further controls and experimentation are necessary to prove this point. Indeed, if antibody is involved in protection, mechanisms by which they protect require investigation. The authors did not detect secretory IgA in vaginal tissue. Instead, they detected IgG that persisted throughout the observational period. Although IgG can be the predominant isotype in the vagina, does IgG prevent an interaction of the fungus with the vaginal epithelium? In interesting previous work, the authors determined that their protective monoclonal antibody, 2G8, which is specific for β-1,3-glucan, directly inhibits fungal growth and adherence [46••]. It remains to be seen whether such antibodies are produced in response to the vaccine and also somehow show up in vaginal fluid. The authors speculate further that, given the inflammatory response in vaginal tissue during Candida vaginitis, opsonic antibodies also may play a role. However, as indicated earlier in this review, the response of inflammatory cells to the vaginal epithelium not only seems to be ineffective in countering the fungi in the vagina, but may be responsible for disease symptoms. Finally, if a protective antibody response to a vaccine is to be clinically relevant or useful, it must show protection that is durable. A sustained memory cell response needs to be shown in future experiments.
Conclusions
This latest vaccination attempt, like those by previous investigators, showed only modest levels of protection and had limitations both in the experimental approach designed to prove protection due to antibody, and in the imaging system because the method requires a genetically manipulated challenge strain. Intuitively, the modest protection in the mouse model should preclude clinical trials until a more consistent and effective vaccine formulation is defined. A caveat, however, is that humans may well respond more robustly and effectively than mice against the vaccine, as was the case during development of anticapsular antibodies against Streptococcus pneumoniae (i.e., precursor work to our present-day pneumococcal vaccines). As elucidated so nicely by Casadevall and Scharff [47], early mouse experiments provided, at times, only marginal protection against pneumococcal disease in the mouse, and led some to conclude that antibodies against the bacterium would not be protective in humans. With that in mind, the anti-Candida vaccine described here may be worth putting into human clinical trials, although the argument can always be made for further development. For women susceptible to RVVC, in whom the vaccine likely would be used, a significant and consistent response to Candida exposure would be required to eliminate or reduce the acute inflammatory response initiated by the vaginal epithelium. If mediated by β-glucan antibodies, such antibodies would need to be present continuously to respond to a low number of Candida which, in the absence of protective antibodies, can initiate inflammation and, thus, symptoms. Based on these criteria, we predict that this vaccine candidate alone would not be effective in the RVCC-susceptible patient. Indeed, it seems appropriate at this time to promote consideration of a multiple vaccine approach, comprised of one or more vaccine candidates reported by others, including SAPs, mannan, and Als3, among others (Table 1).
Taking into account all the known information presented herein, the million dollar question is: How close are we to a vaccine against Candida vaginitis? Ideally, more research is needed to uncover remaining mysteries in the immunopathogenesis of this disease and any genetic associations. Many of the enigmas have been alluded to above, but if a vaccine will afford protection by antibody, additional questions remain. Which route of administration will be optimal for antibody-mediated disease resistance? How does serum antibody titer relate to protection, and how does antibody from the circulation, especially non-IgA antibody classes, track to the vaginal epithelium? Depending on the mechanism of antibody protection, antibody titer, class or sub-class of antibody produced, and antibody specificity are all important considerations, not only for protection, but also for any deleterious effects on the host. If complement and phagocytic cells are involved in protection, the question remains whether these host factors are present in appropriate amounts and location to effect protection. Some of these issues may be circumvented by use of a vaccine that induces antibodies directly cytotoxic for the fungus. However, of fundamental importance is the durability of the host response to the vaccine. Although it is possible that a vaccine can be arrived at empirically, as demonstrated historically for other vaccines, considering the level of current research capability, it is our opinion that answers to many of these fundamental questions should be resolved before embarking on clinical trials.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sobel JD, Faro S, Force R, et al.: Vulvovaginal candidiasis: Epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol 1998, 178:203–211.
Sobel JD: Pathogenesis and epidemiology of vulvovaginal candidiasis. Ann N Y Acad Sci 1988, 544:547–557.
Sobel JD: Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin Infect Dis 1992, 14:S148–S153.
Fidel PL Jr, Sobel JD: Immunopathogenesis of recurrent vulvovaginal candidiasis. Clinical Microbiol Rev 1996, 9:335–348.
Cutler JE, Deepe GS, Jr, Klein BS: Advances in combating fungal diseases: vaccines on the threshold. Nat Rev Microbiol 2007, 5:13–28.
Witkin SS: Inhibition of Candida-induced lymphocyte proliferation by antibody to Candida albicans. Obstet Gynecol 1986, 68:696–699.
Hobbs JR, Briden D, Davidson F, et al.: Immunological aspects of candidal vaginitis. Proc R Soc Med 1977, 70:11–14.
Fong IW, McCleary P, Read S: Cellular immunity of patients with recurrent or refractory vulvovaginal moniliasis. Am J Obstet Gynecol 1992, 166:887–890.
Mendling W, Koldovsky U: Investigations by cell-mediated immunologic tests and therapeutic trials with thymopentin in vaginal mycoses. Infect Dis Obstet Gynecol 1996, 4:225–231.
Fidel PL, Jr, Lynch ME, Redondo-Lopez V, et al.: Systemic cell-mediated immune reactivity in women with recurrent vulvovaginal candidiasis (RVVC). J Infect Dis 1993, 168:1458–1465.
Fidel PL, Jr, Lynch ME, Sobel JD: Effects of preinduced Candida-specific systemic cell-mediated immunity on experimental vaginal candidiasis. Infect Immun 1994, 62:1032–1038.
Fidel PL, Jr, Lynch ME, Conaway DH, et al.: Mice immunized by primary vaginal C. albicans infection develop acquired vaginal mucosal immunity. Inf Immun 1995, 63:547–553.
Hector RF, Domer JE, Carrow EW: Immune responses to Candida albicans in genetically distinct mice. Infect Immun 1982, 38:1020–1028.
Fidel PL, Jr, Lynch ME, Sobel JD: Circulating CD4 and CD8 T cells have little impact on host defense against experimental vaginal candidiasis. Infect Immun 1995, 63:2403–2408.
Black CA, Eyers FM, Dunkley ML, et al.: Major histocompatibility haplotype does not impact the course of experimentally induced murine vaginal candidiasis. Lab Animal Sci 1999, 49:668–672.
Black CA, Eyers FM, Russell A, et al.: Increased severity of Candida vaginitis in BALB/c nu/nu mice versus the parent strain is not abrogated by adoptive transfer of T cell enriched lymphocytes. J Reprod Immunol 1999, 45:1–18.
Nawrot U, Grzybek-Hryncewicz K, Zielska U, et al.: The study of cell-mediated immune response in recurrent vulvovaginal candidiasis [In Process Citation]. FEMS Immunol Med Microbiol 2000, 29:89–94.
Corrigan EM, Clancy RL, Dunkley ML, et al.: Cellular immunity in recurrent vulvovaginal candidiasis. Clin Exp Immunol 1998, 111:574–578.
Fidel PL, Jr, Luo W, Steele C, et al.: Analysis of vaginal cell populations during experimental vaginal candidiasis. Infect Immun 1999, 67:3135–3140.
Taylor BN, Saavedra M, Fidel PL, Jr.: Local Th1/Th2 cytokine production during experimental vaginal candidiasis. Med Mycol 2000, 38:419–431.
Dunbar PR, Hill J, Neale TJ, et al.: Neopterin measurement provides evidence of altered cell-mediated immunity in patients with depression, but not with schizophrenia. Psycho Med 1992, 22:1051–1057.
Cenci E, Mencacci A, Spaccapelo R, et al.: T helper cell type 1 (Th1)- and Th2-like responses are present in mice with gastric candidiasis but protective immunity is associated with Th1 development. J Infect Dis 1995, 171:1279–1288.
Fidel PL, Jr, Barousse M, Espinosa T, et al.: A live intravaginal Candida challenge in humans reveals new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect Immun 2004, 72:2939–2946.
• Yano J, Lilly E, Barousse M, et al.: Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infect Immun 2010, in press. This article describes the novel discovery of the role of calcium-binding proteins in the acute inflammatory response by neutrophils during Candida vaginitis.
Wormley FL, Jr, Steele C, Wozniak K, et al.: Resistance of TCR δ−chain knock-out mice to experimental Candida vaginitis. Infect Immun 2001, 69:7162–7164.
LeBlanc DM, Barousse MM, Fidel PL, Jr: A role for dendritic cells in immunoregulation during experimental vaginal candidiasis. Infect Immun 2006, 74:3213–3221.
Santoni G, Boccanera M, Adriani D, et al.: Immune cell-mediated protection against vaginal candidiasis: Evidence for a major role of vaginal CD4(+) T cells and possible participation of other local lymphocyte effectors. Infect Immun 2002, 70:4791–4797.
De Bernardis F, Santoni G, Boccanera M, et al.: Protection against rat vaginal candidiasis by adoptive transfer of vaginal B lymphocytes. FEMS Yeast Res 2010, 10:432–440.
Cassone A, Boccanera M, Adriani DA, et al.: Rats clearing a vaginal infection by Candida albicans aquire specific, antibody-mediated resistance to vaginal infection. Infect Immun 1995, 63:2619–2624.
De Bernardis F, Boccanera M, Adriana D, et al.: Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect Immun 1997, 65:3399–3405.
Mathur S, Virella G, Koistinen J, et al.: Humoral immunity in vaginal candidiasis. Infect Immun 1977, 15:287–294.
Wozniak KL, Wormley FL, Jr, Fidel PL, Jr.: Candida-specific antibodies during experimental vaginal candidiasis in mice. Infect Immun 2002, 70:5790–5799.
Han Y, Morrison RP, Cutler JE: A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect Immun 1998, 66:5771–5776.
De Bernardis F, Boccanera M, Adriani D, et al.: Intravaginal and intranasal immunizations are equally effective in inducing vaginal antibodies and conferring protection against vaginal candidiasis. Infect Immun 2002, 70:2725–2729.
Han Y, Riesselman MH, Cutler JE: Protection against candidiasis by an immunoglobulin G3 (IgG3) monoclonal antibody specific for the same mannotriose as an IgM protective antibody. Infect Immun 2000, 68:1649–1654.
Han Y, Kozel TR, Zhang MX, et al.: Complement is essential for protection by an IgM and an IgG3 monoclonal antibody against experimental, hematogenously disseminated candidiasis. J Immunol 2001, 167:1550–1557.
Rahman D, Mistry M, Thavaraj S, et al.: Murine model of concurrent oral and vaginal Candida albicans colonization to study epithelial host-pathogen interactions. Microbes Infect 2007, 9:615–622.
Polonelli L, De Bernardis F, Conti S, et al.: Idiotypic intravaginal vaccination to protect against candidal vaginitis by secretory,yeast killer toxin-like anti-idiotypic antibodies. J Immunol 1994, 152:3175–3182.
Spellberg BJ, Ibrahim AS, Avanesian V, et al.: Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J Infect Dis 2006, 194:256–260.
•• Raska M, Belakova J, Horynova M, et al.: Systemic and mucosal immunization with Candida albicans hsp90 elicits hsp90-specific humoral response in vaginal mucosa which is further enhanced during experimental vaginal candidiasis. Med Mycol 2008, 46:411–420. This article describes partial protection against vaginitis following immunization with C. albicans heat shock protein 90
Torosantucci A, Bromuro C, Chiani P, et al.: A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med 2005, 202:597–606.
Moragues MD, Omaetxebarria MJ, Elguezabal N, et al.: A monoclonal antibody directed against a Candida albicans cell wall mannoprotein exerts three anti-C. albicans activities. Infect Immun 2003, 71:5273–5279.
•• Pietrella D, Rachini A, Torosantucci A, et al.: A beta-glucan-conjugate vaccine and anti-beta-glucan antibodies are effective against murine vaginal candidiasis as assessed by a novel in vivo imaging technique. Vaccine 2010, 28:1717–1725. This article describes partial protection against vaginitis in mice vaccinated with β-glucan conjugate vaccine and evaluated by a novel live animal imaging technique.
Moreno-Ruiz E, Ortu G, de Groot PW, et al.: The GPI-modified proteins Pga59 and Pga62 of Candida albicans are required for cell wall integrity. Microbiol 2009, 155:2004–2020.
Enjalbert B, Rachini A, Vediyappan G, et al.: A multifunctional, synthetic Caussia princeps luciferase reporter for live imaging of Candida albicans infections. Infect Immun 2009, 77:4847–4858.
•• Torosantucci A, Chiani P, Bromuro C, et al.: Protection by anti-beta-glucan antibodies is associated with restricted beta-1,3 glucan binding specificity and inhibition of fungal growth and adherence. PLoS ONE 2009, 4:e5392. The mechanism of protection by anti-β-glucan antibodies is uncovered.
Casadevall A, Scharff MD: Serum therapy revisited: animal models of infection and development of passive antibody therapy. Antimicrob Agents Chemother 1994, 38:1695–1702.
Mencacci A, Torosantucci A, Spaccapelo R, et al.: A mannoprotein constituent of Candida albicans that elicits different levels of delayed-type hypersensitivity, cytokine production, and anticandidal protection in mice. Infect Immun 1994, 62:5353–5360.
•• Spellberg B, Ibrahim AS, Lin L, et al.: Antibody titer threshold predicts anti-candidal vaccine efficacy even though the mechanism of protection is induction of cell-mediated immunity. J Infect Dis 2008, 197:967–971. Partial protection against vaginitis is shown with ALS3 vaccine candidate.
Disclosure
No potential conflict of interest relevant to this article was reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fidel, P.L., Cutler, J.E. Prospects for Development of a Vaccine to Prevent and Control Vaginal Candidiasis. Curr Infect Dis Rep 13, 102–107 (2011). https://doi.org/10.1007/s11908-010-0143-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11908-010-0143-y