Abstract
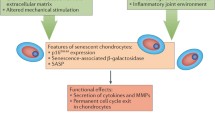
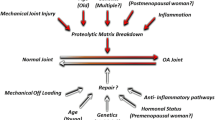
It is well accepted that aging is one of the most prominent risk factors for the initiation and progression of osteoarthritis. One of the most pronounced age-related changes in chondrocytes is the exhibition of a senescent phenotype, which is the result of several factors including the accumulation of reactive oxygen species and advanced glycation end products. Compared with a normal chondrocyte, senescent chondrocytes exhibit an impaired ability to respond to many mechanical and inflammatory insults to the articular cartilage. Furthermore, protein secretion is altered in aging chondrocytes, demonstrated by a decrease in anabolic activity and increased production of proinflammatory cytokines and matrix-degrading enzymes. Together, these events may make the articular cartilage matrix more susceptible to damage and lead to the onset of osteoarthritis. A better understanding of the mechanisms underlying age-related chondrocyte pathophysiology may be critical for the development of novel therapeutic interventions for progressive joint diseases.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cheng HJ, Hootman JM, Murphy LB, Langmaid GA, Helmick CG. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation—United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2010;59:1261–5.
Martin JA, Buckwalter JA. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology. 2002;3:257–64.
Martin JA, Buckwalter JA. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J Bone Joint Surg Am. 2003;85-A Suppl 2:106–10.
Buckwalter JA, Roughley PJ, Rosenberg LC. Age-related changes in cartilage proteoglycans: quantitative electron microscopic studies. Microsc Res Tech. 1994;28:398–408.
Bolton MC, Dudhia J, Bayliss MT. Age-related changes in the synthesis of link protein and aggrecan in human articular cartilage: implications for aggregate stability. Biochem J. 1999;337(Pt 1):77–82.
Fortier LA, Barker JU, Strauss EJ et al. The Role of Growth Factors in Cartilage Repair. Clin Orthop Relat Res 2011.
Loeser RF, Shanker G, Carlson CS, et al. Reduction in the chondrocyte response to insulin-like growth factor 1 in aging and osteoarthritis: studies in a non-human primate model of naturally occurring disease. Arthritis Rheum. 2000;43:2110–20.
Martin JA, Ellerbroek SM, Buckwalter JA. Age-related decline in chondrocyte response to insulin-like growth factor-I: the role of growth factor binding proteins. J Orthop Res. 1997;15:491–8.
Chubinskaya S, Kumar B, Merrihew C, et al. Age-related changes in cartilage endogenous osteogenic protein-1 (OP-1). Biochim Biophys Acta. 2002;1588:126–34.
Blaney Davidson EN, Scharstuhl A, Vitters EL, et al. Reduced transforming growth factor-beta signaling in cartilage of old mice: role in impaired repair capacity. Arthritis Res Ther. 2005;7:R1338–47.
Scharstuhl A, van Beuningen HM, Vitters EL, et al. Loss of transforming growth factor counteraction on interleukin 1 mediated effects in cartilage of old mice. Ann Rheum Dis. 2002;61:1095–8.
van Beuningen HM, van der Kraan PM, Arntz OJ, et al. Transforming growth factor-beta 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab Invest. 1994;71:279–90.
van der Kraan PM, Blaney Davidson EN, van den Berg WB. A role for age-related changes in TGFbeta signaling in aberrant chondrocyte differentiation and osteoarthritis. Arthritis Res Ther. 2010;12:201.
Verzijl N, DeGroot J, Thorpe SR, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275:39027–31.
• Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911–16 e12. This is a database of how various food preparation methods affect levels of AGE formation in food.
Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–21.
• Nah SS, Choi IY, Lee CK, et al. Effects of advanced glycation end products on the expression of COX-2, PGE2 and NO in human osteoarthritic chondrocytes. Rheumatology (Oxford). 2008;47:425–31. This is a study determining the proinflammatory mechanisms of AGEs on chondrocytes.
Nah SS, Choi IY, Yoo B, et al. Advanced glycation end products increases matrix metalloproteinase-1, -3, and −13, and TNF-alpha in human osteoarthritic chondrocytes. FEBS Lett. 2007;581:1928–32.
Huang CY, Lai KY, Hung LF et al. Advanced glycation end products cause collagen II reduction by activating Janus kinase/signal transducer and activator of transcription 3 pathway in porcine chondrocytes. Rheumatology (Oxford) 2011.
Verzijl N, DeGroot J, Ben ZC, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46:114–23.
Chen AC, Temple MM, Ng DM, et al. Induction of advanced glycation end products and alterations of the tensile properties of articular cartilage. Arthritis Rheum. 2002;46:3212–7.
Bank RA, Bayliss MT, Lafeber FP, et al. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998;330(Pt 1):345–51.
Guimaraes EL, Empsen C, Geerts A, et al. Advanced glycation end products induce production of reactive oxygen species via the activation of NADPH oxidase in murine hepatic stellate cells. J Hepatol. 2010;52:389–97.
Yang K, Wang XQ, He YS, et al. Advanced glycation end products induce chemokine/cytokine production via activation of p38 pathway and inhibit proliferation and migration of bone marrow mesenchymal stem cells. Cardiovasc Diabetol. 2010;9:66.
Whaley-Connell A, McCullough PA, Sowers JR. The role of oxidative stress in the metabolic syndrome. Rev Cardiovasc Med. 2011;12:21–9.
Avery SV. Molecular targets of oxidative stress. Biochem J. 2011;434:201–10.
Roberts RA, Laskin DL, Smith CV, et al. Nitrative and oxidative stress in toxicology and disease. Toxicol Sci. 2009;112:4–16.
Studer R, Jaffurs D, Stefanovic-Racic M, et al. Nitric oxide in osteoarthritis. Osteoarthritis Cartilage. 1999;7:377–9.
Hiran TS, Moulton PJ, Hancock JT. Detection of superoxide and NADPH oxidase in porcine articular chondrocytes. Free Radic Biol Med. 1997;23:736–43.
Tiku ML, Shah R, Allison GT. Evidence linking chondrocyte lipid peroxidation to cartilage matrix protein degradation. Possible role in cartilage aging and the pathogenesis of osteoarthritis. J Biol Chem. 2000;275:20069–76.
Jallali N, Ridha H, Thrasivoulou C, et al. Vulnerability to ROS-induced cell death in ageing articular cartilage: the role of antioxidant enzyme activity. Osteoarthritis Cartilage. 2005;13:614–22.
• Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol. 2011;7:161–9. This is a review on the role mitochondrial dysfunction plays in the pathogenesis of OA.
Davies CM, Guilak F, Weinberg JB, et al. Reactive nitrogen and oxygen species in interleukin-1-mediated DNA damage associated with osteoarthritis. Osteoarthritis Cartilage. 2008;16:624–30.
Yudoh K, Nguyen T, Nakamura H, et al. Potential involvement of oxidative stress in cartilage senescence and development of osteoarthritis: oxidative stress induces chondrocyte telomere instability and downregulation of chondrocyte function. Arthritis Res Ther. 2005;7:R380–91.
Nakagawa S, Arai Y, Mazda O, et al. N-acetylcysteine prevents nitric oxide-induced chondrocyte apoptosis and cartilage degeneration in an experimental model of osteoarthritis. J Orthop Res. 2010;28:156–63.
Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med. 2004;37:768–84.
Marie PJ, Kassem M. Extrinsic mechanisms involved in age-related defective bone formation. J Clin Endocrinol Metab. 2011;96:600–9.
Shirazi R, Shirazi-Adl A. Computational biomechanics of articular cartilage of human knee joint: effect of osteochondral defects. J Biomech. 2009;42:2458–65.
Stevens JE, Binder-Macleod S, Snyder-Mackler L. Characterization of the human quadriceps muscle in active elders. Arch Phys Med Rehabil. 2001;82:973–8.
Sun HB. Mechanical loading, cartilage degradation, and arthritis. Ann N Y Acad Sci. 2010;1211:37–50.
Chang BD, Broude EV, Fang J, et al. p21Waf1/Cip1/Sdi1-induced growth arrest is associated with depletion of mitosis-control proteins and leads to abnormal mitosis and endoreduplication in recovering cells. Oncogene. 2000;19:2165–70.
• Shimada H, Sakakima H, Tsuchimochi K, et al. Senescence of chondrocytes in aging articular cartilage: GADD45beta mediates p21 expression in association with C/EBPbeta in senescence-accelerated mice. Pathol Res Pract. 2011;207:225–31. This is an in vivo model of aging used to elucidate mechanisms of chondrocyte senescence.
Hoffman B, Liebermann DA. Gadd45 modulation of intrinsic and extrinsic stress responses in myeloid cells. J Cell Physiol. 2009;218:26–31.
Martos-Rodriguez A, Santos-Alvarez I, Campo-Ruiz V, et al. Expression of CCAAT/enhancer-binding protein-beta (C/EBPbeta) and CHOP in the murine growth plate. Two possible key modulators of chondrocyte differentiation. J Bone Joint Surg Br. 2003;85:1190–5.
Xin H, Liu D, Songyang Z. The telosome/shelterin complex and its functions. Genome Biol. 2008;9:232.
Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–34.
Thacker J, Zdzienicka MZ. The XRCC genes: expanding roles in DNA double-strand break repair. DNA Repair (Amst). 2004;3:1081–90.
Guarente L. Diverse and dynamic functions of the Sir silencing complex. Nat Genet. 1999;23:281–5.
• Brandl A, Hartmann A, Bechmann V, et al. Oxidative stress induces senescence in chondrocytes. J Orthop Res. 2011;29:1114–20. This paper shows molecular mechanisms mediating the effects of oxidative stress on chondrocyte senescence.
Park WY, Park JS, Cho KA, et al. Up-regulation of caveolin attenuates epidermal growth factor signaling in senescent cells. J Biol Chem. 2000;275:20847–52.
Dai SM, Shan ZZ, Nakamura H, et al. Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: possible involvement of caveolin 1-induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis. Arthritis Rheum. 2006;54:818–31.
Yudoh K, Shi Y, Karasawa R. Angiogenic growth factors inhibit chondrocyte ageing in osteoarthritis: potential involvement of catabolic stress-induced overexpression of caveolin-1 in cellular ageing. Int J Rheum Dis. 2009;12:90–9.
Aigner T, Hemmel M, Neureiter D, et al. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 2001;44:1304–12.
Horton Jr WE, Feng L, Adams C. Chondrocyte apoptosis in development, aging and disease. Matrix Biol. 1998;17:107–15.
Loeser RF, Pacione CA, Chubinskaya S. The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003;48:2188–96.
Allende JE, Allende CC. Protein kinases. 4. Protein kinase CK2: an enzyme with multiple substrates and a puzzling regulation. FASEB J. 1995;9:313–23.
Lee SW, Song YS, Lee SY, et al. Downregulation of protein kinase CK2 activity facilitates tumor necrosis factor-alpha-mediated chondrocyte death through apoptosis and autophagy. PLoS One. 2011;6:e19163.
Taniguchi N, Carames B, Ronfani L, et al. Aging-related loss of the chromatin protein HMGB2 in articular cartilage is linked to reduced cellularity and osteoarthritis. Proc Natl Acad Sci U S A. 2009;106:1181–6.
Zhou Z, Akinbiyi T, Xu L, et al. Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell. 2010;9:911–5.
Leong DJ, Li YH, Gu XI, et al. Physiological loading of joints prevents cartilage degradation through CITED2. FASEB J. 2011;25:182–91.
Yokota H, Goldring MB, Sun HB. CITED2-mediated regulation of MMP-1 and MMP-13 in human chondrocytes under flow shear. J Biol Chem. 2003;278:47275–80.
Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–46.
Hinton R, Moody RL, Davis AW, et al. Osteoarthritis: diagnosis and therapeutic considerations. Am Fam Physician. 2002;65:841–8.
Sinkov V, Cymet T. Osteoarthritis: understanding the pathophysiology, genetics, and treatments. J Natl Med Assoc. 2003;95:475–82.
Peat G, Croft P, Hay E. Clinical assessment of the osteoarthritis patient. Best Pract Res Clin Rheumatol. 2001;15:527–44.
Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3:107–13.
Fukui N, Ikeda Y, Ohnuki T, et al. Regional differences in chondrocyte metabolism in osteoarthritis: a detailed analysis by laser capture microdissection. Arthritis Rheum. 2008;58:154–63.
Visco DM, Johnstone B, Hill MA, et al. Immunohistochemical analysis of 3-B-(−) and 7-D-4 epitope expression in canine osteoarthritis. Arthritis Rheum. 1993;36:1718–25.
•• Loeser RF. Age-related changes in the musculoskeletal system and the development of osteoarthritis. Clin Geriatr Med. 2010;26:371–86. This is a review on the effect of aging joint tissues on the development of OA.
Traustadottir T, Davies SS, Su Y, et al. Oxidative stress in older adults: effects of physical fitness. Age (Dordr) 2011.
Boor P, Celec P, Behuliak M, et al. Regular moderate exercise reduces advanced glycation and ameliorates early diabetic nephropathy in obese Zucker rats. Metabolism. 2009;58:1669–77.
Acknowledgment
The author thanks the National Institutes of Health for funding this work (AR52743 and AR47628).
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leong, D.J., Sun, H.B. Events in Articular Chondrocytes with Aging. Curr Osteoporos Rep 9, 196–201 (2011). https://doi.org/10.1007/s11914-011-0070-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-011-0070-3