Abstract
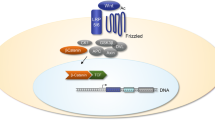
Osteoporosis and osteoarthritis are common musculoskeletal disorders in which cause and outcome are determined by genetic and environmental factors. The WNT signaling pathway plays an important role in skeletal development and growth. Polymorphisms in a number of genes that belong to this pathway are associated with osteoarthritis and/or osteoporosis. This suggests a role for this molecular signaling pathway in postnatal joint and bone homeostasis and pathology. Increased activity of WNT signaling strengthens the bone but may have adverse effects on the articular cartilage. Frizzled related protein (FRZB) plays a role in both bone and cartilage. Better understanding of the WNT pathway and its modulators may lead to specific therapeutics for both osteoarthritis and osteoporosis. This review focuses on recent studies in human genetics and animal models and highlights the potential clinical relevance of this rapidly evolving field of research.
Similar content being viewed by others
References and Recommended Reading
Gordon MD, Nusse R: Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem 2006, 281:22429–22433.
Tu X, Joeng KS, Nakayama KI, et al.: Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev Cell 2007, 12:113–127.
Gong Y, Slee RB, Fukai N, et al.: LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 2001, 107:513–523.
MacDonald BT, Joiner DM, Oyserman SM, et al.: Bone mass is inversely proportional to Dkk1 levels in mice. Bone 2007, 41:331–339.
Balemans W, Van Hul W: The genetics of low-density lipoprotein receptor-related protein 5 in bone: a story of extremes. Endocrinology 2007, 148:2622–2629.
Richards JB, Rivadeneira F, Inouye M, et al.: Bone mineral density, osteoporosis, and osteoporotic fractures: a genomewide association study. Lancet 2008, 371:1505–1512.
van Meurs JB, Trikalinos TA, Ralston SH, et al.: Largescale analysis of association between LRP5 and LRP6 variants and osteoporosis. JAMA 2008, 299:1277–1290.
Grundberg E, Lau EM, Lorentzson M, et al.: Large-scale association study between two coding LRP5 gene polymorphisms and bone phenotypes and fractures in men. Osteoporos Int 2007, 19:829–837.
van Meurs JB, Rivadeneira F, Jhamai M, et al.: Common genetic variation of the low-density lipoprotein receptor-related protein 5 and 6 genes determines fracture risk in elderly white men. J Bone Miner Res 2006, 21:141–150.
Kaufman JM, Ostertag A, Saint-Pierre A, et al.: Genomewide linkage screen of bone mineral density (BMD) in European pedigrees ascertained through a male relative with low BMD values: Evidence for Quantitative Trait Loci on 17q21-23, 11q12-13, 13q12-14 and 22q11. J Clin Endocrinol Metab 2008 (in press).
Kiel DP, Demissie S, Dupuis J, et al.: Genome-wide association with bone mass and geometry in the Framingham Heart Study. BMC Med Genet 2007, 8(Suppl 1):S14.
Kato M, Patel MS, Levasseur R, et al.: Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol 2002, 157:303–314.
Akhter MP, Wells DJ, Short SJ, et al.: Bone biomechanical properties in LRP5 mutant mice. Bone 2004, 35:162–169.
Babij P, Zhao W, Small C, et al.: High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res 2003, 18:960–974.
Holmen SL, Giambernardi TA, Zylstra CR, et al.: Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res 2004, 19:2033–2040.
Bodine PV, Zhao W, Kharode YP, et al.: The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol 2004, 18:1222–1237.
Lories RJ, Peeters J, Bakker A, et al.: Articular cartilage and biomechanical properties of the long bones in Frzbknockout mice. Arthritis Rheum 2007, 56:4095–4103.
Bodine PV, Billiard J, Moran RA, et al.: The Wnt antagonist secreted frizzled-related protein-1 controls osteoblast and osteocyte apoptosis. J Cell Biochem 2005, 96:1212–1230.
Hausler KD, Horwood NJ, Chuman Y, et al.: Secreted frizzled-related protein-1 inhibits RANKL-dependent osteoclast formation. J Bone Miner Res 2004, 19:1873–1881.
Nakanishi R, Shimizu M, Mori M, et al.: Secreted frizzled-related protein 4 is a negative regulator of peak BMD in SAMP6 mice. J Bone Miner Res 2006, 21:1713–1721.
Nakanishi R, Akiyama H, Kimura H, et al.: Osteoblast-targeted expression of Sfrp4 in mice results in low bone mass. J Bone Miner Res 2008, 23:271–277.
Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, et al.: Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell 2001, 1:423–434.
Li X, Liu P, Liu W, et al.: Dkk2 has a role in terminal osteoblast differentiation and mineralized matrix formation. Nat Genet 2005, 37:945–952.
Morvan F, Boulukos K, Clement-Lacroix P, et al.: Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Miner Res 2006, 21:934–945.
Sawakami K, Robling AG, Ai M, et al.: The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem 2006, 281:23698–23711.
Lau HH, Ng MY, Cheung WM, et al.: Assessment of linkage and association of 13 genetic loci with bone mineral density. J Bone Miner Metab 2006, 24:226–234.
Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, et al.: Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem 2006, 281:31720–31728.
Damien E, Price JS, Lanyon LE: Mechanical strain stimulates osteoblast proliferation through the estrogen receptor in males as well as females. J Bone Miner Res 2000, 15:2169–2177.
Zaman G, Jessop HL, Muzylak M, et al.: Osteocytes use estrogen receptor alpha to respond to strain but their ERalpha content is regulated by estrogen. J Bone Miner Res 2006, 21:1297–1306.
Armstrong VJ, Muzylak M, Sunters A, et al.: Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J Biol Chem 2007, 282:20715–20727.
Foo C, Frey S, Yang HH, et al.: Downregulation of beta-catenin and transdifferentiation of human osteoblasts to adipocytes under estrogen deficiency. Gynecol Endocrinol 2007, 23:535–540.
Ohnaka K, Taniguchi H, Kawate H, et al.: Glucocorticoid enhances the expression of dickkopf-1 in human osteoblasts: novel mechanism of glucocorticoid-induced osteoporosis. Biochem Biophys Res Commun 2004, 318:259–264.
Smith E, Frenkel B: Glucocorticoids inhibit the transcriptional activity of LEF/TCF in differentiating osteoblasts in a glycogen synthase kinase-3beta-dependent and -independent manner. J Biol Chem 2005, 280:2388–2394.
Hurson CJ, Butler JS, Keating DT, et al.: Gene expression analysis in human osteoblasts exposed to dexamethasone identifies altered developmental pathways as putative drivers of osteoporosis. BMC Musculoskelet Disord 2007, 8:12.
Yao W, Cheng Z, Busse C, et al.: Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: a longitudinal study of gene expression in bone tissue from glucocorticoid-treated mice. Arthritis Rheum 2008, 58:1674–1686.
Valdes AM, Doherty M, Spector TD: The additive effect of individual genes in predicting risk of knee osteoarthritis. Ann Rheum Dis 2008, 67:124–127.
Loughlin J, Dowling B, Chapman K, et al.: Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci U S A 2004, 101:9757–9762.
Min JL, Meulenbelt I, Riyazi N, et al.: Association of the Frizzled-related protein gene with symptomatic osteoarthritis at multiple sites. Arthritis Rheum 2005, 52:1077–1080.
Lories RJ, Boonen S, Peeters J, et al.: Evidence for a differential association of the Arg200Trp single-nucleotide polymorphism in FRZB with hip osteoarthritis and osteoporosis. Rheumatology (Oxford) 2006, 45:113–114.
Lane NE, Lian K, Nevitt MC, et al.: Frizzled-related protein variants are risk factors for hip osteoarthritis. Arthritis Rheum 2006, 54:1246–1254.
Valdes AM, Loughlin J, Oene MV, et al.: Sex and ethnic differences in the association of ASPN, CALM1, COL2A1, COMP, and FRZB with genetic susceptibility to osteoarthritis of the knee. Arthritis Rheum 2007, 56:137–146.
Kerkhof JM, Uitterlinden AG, Valdes AM, et al.: Radiographic osteoarthritis at three joint sites and FRZB, LRP5, and LRP6 polymorphisms in two population-based cohorts. Osteoarthritis Cartilage 2008 (in press).
Smith AJ, Gidley J, Sandy JR, et al.: Haplotypes of the low-density lipoprotein receptor-related protein 5 (LRP5) gene: are they a risk factor in osteoarthritis? Osteoarthritis Cartilage 2005, 13:608–613.
Urano T, Shiraki M, Narusawa K, et al.: Q89R polymorphism in the LDL receptor-related protein 5 gene is associated with spinal osteoarthritis in postmenopausal Japanese women. Spine 2007, 32:25–29.
Dell’Accio F, De Bari C, El Tawil NM, et al.: Activation of WNT and BMP signaling in adult human articular cartilage following mechanical injury. Arthritis Res Ther 2006, 8:R139.
Dell’Accio F, De Bari C, Eltawil NM, et al.: Identification of the molecular response of articular cartilage to injury, by microarray screening: Wnt-16 expression and signaling after injury and in osteoarthritis. Arthritis Rheum 2008, 58:1410–1421.
Appleton CT, Pitelka V, Henry J, Beier F: Global analyses of gene expression in early experimental osteoarthritis. Arthritis Rheum 2007, 56:1854–1868.
Zhu M, Chen M, Zuscik M, et al.: Inhibition of betacatenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum 2008, 58:2053–2064.
Dequeker J, Aerssens J, Luyten FP: Osteoarthritis and osteoporosis: clinical and research evidence of inverse relationship. Aging Clin Exp Res 2003, 15:426–439.
Lane NE, Nevitt MC, Lui LY, et al.: Wnt signaling antagonists are potential prognostic biomarkers for the progression of radiographic hip osteoarthritis in elderly Caucasian women. Arthritis Rheum 2007, 56:3319–3325.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lodewyckx, L., Lories, R.J.U. WNT signaling in osteoarthritis and osteoporosis: What is the biological significance for the clinician?. Curr Rheumatol Rep 11, 23–30 (2009). https://doi.org/10.1007/s11926-009-0004-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-009-0004-6