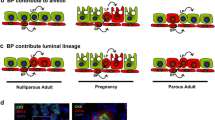
Abstract
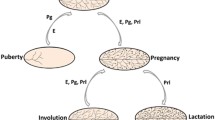
The mammary gland is a unique organ in that it undergoes most of its development after birth under the control of systemic hormones. Whereas in most other organs stem cells divide in response to local stimuli, to replace lost cells, in the mammary gland large numbers of cells need to be generated at specific times during puberty, estrous cycles and pregnancy to generate new tissue structures. This puts special demands on the mammary stem cells and requires coordination of local events with systemic needs. Our aim is to understand how the female reproductive hormones control mammary gland development and influence tumorigenesis. We have shown that steroid hormones act in a paracrine fashion in the mammary gland delegating different functions to locally produced factors. These in turn, affect cell–cell interactions that result in changes of cell behavior required for morphogenesis and differentiation. Here, we discuss how these hormonally regulated paracrine interactions may impinge on stem cells and the stem cell niche and how this integration of signals adds extra levels of complexity to current mammary stem cell models. We propose a model whereby the stem cell niches change depending on the developmental stages and the hormonal milieu. According to this model, repeated hormone stimulation of stem cells and their niches in the course of menstrual cycles may be an important early event in breast carcinogenesis and may explain the conundrum why breast cancer risk increases with the number of menstrual cycles experienced prior to a first pregnancy.




Similar content being viewed by others
References
Daniel, C. W., & Silberstein, G. B. (1987). Postnatal development of the rodent mammary gland. In C. W. Daniel (Ed.), The mammary gland (pp. 3–31). New York: Plenum.
Sakakura, T. (1987). Developmental biology of the mammary gland. In M. C. a. D. Neville C. W. (Ed.), The mammary gland (pp. 37–63). New York: Plenum.
Brisken, C., Kaur, S., Chavarria, T. E., Binart, N., Sutherland, R. L., Weinberg, R. A., et al. (1999). Prolactin controls mammary gland development via direct and indirect mechanisms. Developments in Biologicals, 210(1), 96–106.
DeOme, K. B., Faulkin, L. J., Jr., Bern, H. A., & Blair, P. B. (1959). Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Research, 19, 511–520.
Shackleton, M., Vaillant F., Simpson, K. J., Stingl, J., Smyth, G. K., Asselin-Labat, M. L., et al. (2006). Generation of a functional mammary gland from a single stem cell. Nature, 439(7072), 84–88.
Stingl, J., Eirew, P., Ricketson, I., Shackleton, M., Vaillant, F., Choi, D., et al. (2006). Purification and unique properties of mammary epithelial stem cells. Nature, 439(7079), 993–997.
Sleeman, K. E., Kendrick, H., Robertson, D., Isacke, C. M., Ashworth, A., & Smalley, M. J. (2007). Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. Journal of Cell Biology, 176(1), 19–26.
Asselin-Labat, M. L., Shackleton, M., Stingl, J., Vaillant, F., Forrest, N. C., Eaves, C. J., et al. (2006). Steroid hormone receptor status of mouse mammary stem cells. Journal of the National Cancer Institute, 98(14), 1011–1014.
Chepko, G., Slack, R., Carbott, D., Khan, S., Steadman, L., & Dickson, R. B. (2005). Differential alteration of stem and other cell populations in ducts and lobules of TGFalpha and c-Myc transgenic mouse mammary epithelium. Tissue Cell, 37(5), 393–412.
Chepko, G., & Smith, G. H. (1997). Three division-competent, structurally-distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell, 29(2), 239–253.
Chepko, G., & Smith, G. H. (1999). Mammary epithelial stem cells: Our current understanding. Journal of Mammary Gland Biology and Neoplasia, 4(1), 35–52.
Kordon, E. C., & Smith, G. H. (1998). An entire functional mammary gland may comprise the progeny from a single cell. Development, 125(10), 1921–1930.
Rizvi, A. Z., & Wong, M. H. (2005). Epithelial stem cells and their niche: There’s no place like home. Stem Cells, 23(2), 150–165.
Lin, H. (2002). The stem-cell niche theory: Lessons from flies. Nature Reviews. Genetics, 3(12), 931–940.
Wilson, A., & Trumpp, A. (2006). Bone-marrow haematopoietic-stem-cell niches. Nature Reviews. Immunology, 6(2), 93–106.
Chepko, G., & Dickson, R. B. (2003). Ultrastructure of the putative stem cell niche in rat mammary epithelium. Tissue Cell, 35(2), 83–93.
Boulanger, C. A., Mack, D. L., Booth, B. W., & Smith, G. H. (2007). Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proceedings of the National Academy of Sciences of the United States of America, 104(10), 3871–3876.
Villadsen, R., Fridriksdottir, A. J., Ronnov-Jessen, L., Gudjonsson, T., Rank, F., Labarge, M. A., et al. (2007). Evidence for a stem cell hierarchy in the adult human breast. Journal of Cell Biology, 177(1), 87–101.
Moraes, R. C., Zhang, X., Harrington, N., Fung, J. Y., Wu, M. F., Hilsenbeck, S. G., et al. (2007). Constitutive activation of smoothened (SMO) in mammary glands of transgenic mice leads to increased proliferation, altered differentiation and ductal dysplasia. Development, 134(6), 1231–1242.
Daniel, C. W., Silberstein, G. B., & Strickland, P. (1987). Direct action of 17 beta-estradiol on mouse mammary ducts analyzed by sustained release implants and steroid autoradiography. Cancer Research, 47(22), 6052–6057.
Mallepell, S., Krust, A., Chambon, P., & Brisken, C. (2006). Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proceedings of the National Academy of Sciences of the United States of America, 103(7), 2196–2201.
Stumpf, W. E., Narbaitz, R., & Sar, M. (1980). Estrogen receptors in the fetal mouse. Journal of Steroid Biochemistry, 12, 55–64.
Brisken, C., Heineman, A., Chavarria, T., Elenbaas, B., Tan, J., Dey, S., et al. (2000). Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes and Development, 14(6), 650–654.
Coleman, S., Silberstein, G. B., & Daniel, C. W. (1988). Ductal morphogenesis in the mouse mammary gland: Evidence supporting a role for epidermal growth factor. Developments in Biologicals, 127(2), 304–315.
Ciarloni, L., Mallepell, S., & Brisken, C. (2007). Amphiregulin is an essential mediator of estrogen receptor {alpha} function in mammary gland development. Proceedings of the National Academy of Sciences of the United States of America, 104(13), 5455–5460.
Wiesen, J., Young, P., Werb, Z., & Cunha, G. (1999). Signaling through the stromal epidermal growth factor receptor is necessary for mammary ductal development. Development, 126(2), p335–p344.
Gschwind, A., Hart, S., Fischer, O. M., & Ullrich, A. (2003). TACE cleavage of proamphiregulin regulates GPCR-induced proliferation and motility of cancer cells. EMBO Journal, 22(10), 2411–2421.
Kouros-Mehr, H., & Werb, Z. (2006). Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Developmental Dynamics, 235(12), 3404–3412.
Fata, J. E., Werb, Z., & Bissell, M. J. (2004). Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Research, 6(1), 1–11.
Simian, M., Hirai, Y., Navre, M., Werb, Z., Lochter, A. & Bissell, M. J. (2001). The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development, 128(16), 3117–3131.
Sternlicht, M. D., Kouros-Mehr, H., Lu, P. & Werb, Z. (2006). Hormonal and local control of mammary branching morphogenesis. Differentiation, 74(7), 365–381.
Dontu, G., Abdallah, W. M., Foley, J. M., Jackson, K. W., Clarke, M. F., Kawamura, M. J., et al. (2003). In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes and Development, 17(10), 1253–1270.
Yamaguchi, Y., Mann, D. M., & Ruoslahti, E. (1990). Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature, 346(6281), 281–284.
Derynck, R., & Zhang, Y. E. (2003). Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature, 425(6958), 577–584.
Schwertfeger, K. L., Rosen, J. M., & Cohen, D. A. (2006). Mammary gland macrophages: Pleiotropic functions in mammary development. Journal of Mammary Gland Biology and Neoplasia, 11(3–4), 229–238.
Brisken, C., et al. (1998). A paracrine role for the epithelial progesterone receptor in mammary gland development. Proceedings of the National Academy of Sciences of the United States of America, 95(9), p5076–p5081.
Mulac-Jericevic, B., et al. (2003). Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proceedings of the National Academy of Sciences of the United States of America, 100(17), 9744–9749.
Brisken, C., et al. (2002). IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Developmental Cell, 3(6), 877–887.
Cao, Y., et al. (2001). IKKalpha provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell, 107(6), 763–775.
Willert, K., et al. (2003). Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature, 23(6938), 448–452.
Reya, T., & Clevers, H. (2005). Wnt signalling in stem cells and cancer. Nature, 434(7035), 843–850.
Li, Y., Welm, B., Podsypanina, K., Huang, S., Chamorro, M., Zhang, X. et al. (2003). Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proceedings of the National Academy of Sciences of the United States of America, 100(26), 15853–18858.
Liu, B. Y., McDermott, S. P., Khwaja, S. S., & Alexander, C. M. (2004). The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proceedings of the National Academy of Sciences of the United States of America, 101(12), 4158–4163.
Foley, J., Dann, P., Hong, J., Cosgrove, J., Dreyer, B., Rimm, D., et al. (2001). Parathyroid hormone-related protein maintains mammary epithelial fate and triggers nipple skin differentiation during embryonic breast development. Development, 128(4), 513–525.
Dunbar, M. E., & Wysolmerski, J. J. (1999). Parathyroid hormone-related protein: A developmental regulatory molecule necessary for mammary gland development. Journal of Mammary Gland Biology and Neoplasia, 4(1), 21–34.
Wysolmerski, J. J., Philbrick, W. M., Dunbar, M. E., Lanske, B., Kronenberg, H. & Broadus, A. E. (1998). Rescue of the parathyroid hormone-related protein knockout mouse demonstrates that parathyroid hormone-related protein is essential for mammary gland development. Development, 125(7), 1285–1294.
Hens, J. R., Dann, P., Zhang, J. P., Harris, S., Robinson, G. W., & Wysolmerski, J. (2007). BMP4 and PTHrP interact to stimulate ductal outgrowth during embryonic mammary development and to inhibit hair follicle induction. Development, 134(6), 1221–1230.
Rudland, P. S., Platt-Higgins, A. M., Wilkinson, M. C., & Fernig, D. G. (1993). Immunocytochemical identification of basic fibroblast growth factor in the developing rat mammary gland: Variations in location are dependent on glandular structure and differentiation. Journal of Histochemistry and Cytochemistry, 41(6), 887–898.
Mailleux, A. A., Spencer-Dene, B., Dillon, C., Ndiaye, D., Savona-Baron, C., Itoh, N., et al. (2002). Role of FGF10/FGFR2b signaling during mammary gland development in the mouse embryo. Development, 129(1), 53–60.
van Genderen, C., Okamura, R. M., Farinas, I., Quo, R. G., Parslow, T. G., Bruhn, L., et al. (1994). Development of several organs that require inductive epithelial–mesenchymal interactions is impaired in LEF-1-deficient mice. Genes and Development, 8(22), 2691–2703.
Chu, E. Y., Hens, J., Andl, T., Kairo, A., Yamaguchi, T. P., Brisken, C., et al. (2004). Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development, 131(19), 4819–4829.
Das, S. K., Chakraborty, I., Paria, B. C., Wang, X. N., Plowman,G., & Dey, S. K. (1995). Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Molecular Endocrinology, 9(6), 691–705.
Bocchinfuso, W. P., Hively, W. P., Couse, J. F., Varmus, H. E., & Korach, K. S. (1999). A mouse mammary tumor virus-Wnt-1 transgene induces mammary gland hyperplasia and tumorigenesis in mice lacking estrogen receptor-alpha. Cancer Research, 59(8), 1869–1876.
Wang, J. C., & Dick, J. E. (2005). Cancer stem cells: Lessons from leukemia. Trends in Cell Biology, 15(9), 494–501.
Smalley, M., & Ashworth, A. (2003). Stem cells and breast cancer: A field in transit. Nature Reviews. Cancer, 3(11), 832–844.
Al-Hajj, M., & Clarke, M. F. (2004). Self-renewal and solid tumor stem cells. Oncogene, 23(43), 7274–7282.
Dontu, G., Liu, S., & Wicha, M. S. (2005). Stem cells in mammary development and carcinogenesis: Implications for prevention and treatment. Stem Cell Reviews, 1(3), 207–213.
Pike, M. C., & Ross, R. K. (2000). Progestins and menopause: Epidemiological studies of risks of endometrial and breast cancer. Steroids, 65(10–11), 659–664.
Brennan, K. R., & Brown, A. M. (2004). Wnt proteins in mammary development and cancer. Journal of Mammary Gland Biology and Neoplasia, 9(2), 119–131.
Ugolini, F., Charafe-Jauffret, E., Bardou, V. J., Geneix, J., Adelaide, J., Labat-Moleur, F., et al. (2001). WNT pathway and mammary carcinogenesis: Loss of expression of candidate tumor suppressor gene SFRP1 in most invasive carcinomas except of the medullary type. Oncogene, 20(41), 5810–5817.
Dontu, G., Jackson, K. W., McNicholas, E., Kawamura, M. J., Abdallah, W. M., & Wicha, M. S. (2004). Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Research, 6(6), R605–R615.
Liu, S., Dontu, G., & Wicha, M. S. (2005). Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Research, 7(3), 86–95.
Silberstein, G. B., Van Horn, K., Hrabeta-Robinson, E., & Compton, J. (2006). Estrogen-triggered delays in mammary gland gene expression during the estrous cycle: Evidence for a novel timing system. Journal of Endocrinology, 190(2), 225–239.
Acknowledgements
The authors thank Manfred Beleut for helpful discussions and critical comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brisken, C., Duss, S. Stem Cells and the Stem Cell Niche in the Breast: An Integrated Hormonal and Developmental Perspective. Stem Cell Rev 3, 147–156 (2007). https://doi.org/10.1007/s12015-007-0019-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-007-0019-1