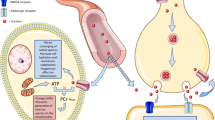
Abstract
Substantial evidence indicates bioenergetic dysfunction and mitochondrial impairment contribute either directly and/or indirectly to the pathogenesis of numerous neurodegenerative disorders. Treatment paradigms aimed at ameliorating this cellular energy deficit and/or improving mitochondrial function in these neurodegenerative disorders may prove to be useful as a therapeutic intervention. Creatine is a molecule that is produced both endogenously, and acquired exogenously through diet, and is an extremely important molecule that participates in buffering intracellular energy stores. Once creatine is transported into cells, creatine kinase catalyzes the reversible transphosphorylation of creatine via ATP to enhance the phosphocreatine energy pool. Creatine kinase enzymes are located at strategic intracellular sites to couple areas of high energy expenditure to the efficient regeneration of ATP. Thus, the creatinekinase/phosphocreatine system plays an integral role in energy buffering and overall cellular bioenergetics. Originally, exogenous creatine supplementation was widely used only as an ergogenic aid to increase the phosphocreatine pool within muscle to bolster athletic performance. However, the potential therapeutic value of creatine supplementation has recently been investigated with respect to various neurodegenerative disorders that have been associated with bioenergetic deficits as playing a role in disease etiology and/or progression which include; Alzheimer’s, Parkinson’s, amyotrophic lateral sclerosis (ALS), and Huntington’s disease. This review discusses the contribution of mitochondria and bioenergetics to the progression of these neurodegenerative diseases and investigates the potential neuroprotective value of creatine supplementation in each of these neurological diseases. In summary, current literature suggests that exogenous creatine supplementation is most efficacious as a treatment paradigm in Huntington’s and Parkinson’s disease but appears to be less effective for ALS and Alzheimer’s disease.





Similar content being viewed by others
References
Adams, J. M., & Cory, S. (1998). The Bcl-2 protein family: Arbiters of cell survival. Science, 281, 1322–1326. doi:10.1126/science.281.5381.1322.
Adhihetty, P. J., & Hood, D. A. (2003). Mechanisms of apoptosis in skeletal muscle. Basic and applied myology, 13, 171–179.
Adhihetty, P. J., Irrcher, I., Joseph, A. M., Ljubicic, V., & Hood, D. A. (2003). Plasticity of skeletal muscle mitochondria in response to contractile activity. Experimental Physiology, 88, 99–107. doi:10.1113/eph8802505.
Aksenov, M., Aksenova, M., Butterfield, D. A., & Markesbery, W. R. (2000). Oxidative modification of creatine kinase BB in Alzheimer’s disease brain. Journal of Neurochemistry, 74, 2520–2527. doi:10.1046/j.1471-4159.2000.0742520.x.
Alston, T. A., Mela, L., & Bright, H. J. (1977). 3-Nitropropionate, the toxic substance of Indigofera, is a suicide inactivator of succinate dehydrogenase. Proceedings of the National Academy of Sciences of the United States of America, 74, 3767–3771. doi:10.1073/pnas.74.9.3767.
Andreassen, O. A., Dedeoglu, A., Ferrante, R. J., Jenkins, B. G., Ferrante, K. L., Thomas, M., et al. (2001). Creatine increase survival and delays motor symptoms in a transgenic animal model of Huntington’s disease. Neurobiology of Disease, 8, 479–491. doi:10.1006/nbdi.2001.0406.
Andres, R. H., Ducray, A. D., Schlattner, U., Wallimann, T., & Widmer, H. R. (2008). Functions and effects of creatine in the central nervous system. Brain Research Bulletin, 76, 329–343. doi:10.1016/j.brainresbull.2008.02.035.
Baines, C. P., Kaiser, R. A., Purcell, N. H., Blair, N. S., Osinska, H., Hambleton, M. A., et al. (2005). Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature, 434, 658–662. doi:10.1038/nature03434.
Baker, S. K., & Tarnopolsky, M. A. (2003). Targeting cellular energy production in neurological disorders. Expert Opinion on Investigational Drugs, 12, 1655–1679. doi:10.1517/13543784.12.10.1655.
Beal, M. F., Brouillet, E., Jenkins, B. G., Ferrante, R. J., Kowall, N. W., Miller, J. M., et al. (1993). Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. Journal of Neuroscience, 13, 4181–4192.
Beal, M. F. (1995). Aging, energy, and oxidative stress in neurodegenerative diseases. Annals of Neurology, 38, 357–366. doi:10.1002/ana.410380304.
Beal, M. F. (1996). Mitochondria, free radicals, and neurodegeneration. Current Opinion in Neurobiology, 6, 661–666. doi:10.1016/S0959-4388(96)80100-0.
Beal, M. F. (2000a). Mitochondria and the pathogenesis of ALS. Brain, 123(Pt 7), 1291–1292. doi:10.1093/brain/123.7.1291.
Beal, M. F. (2000b). Energetics in the pathogenesis of neurodegenerative diseases. Trends in Neurosciences, 23, 298–304. doi:10.1016/S0166-2236(00)01584-8.
Beal, M. F. (2000c). Limited-time exposure to mitochondrial toxins may lead to chronic progressive neurodegenerative diseases. Movement Disorders, 15, 434–435. doi:10.1002/1531-8257(200005)15:3<434::AID-MDS1002>3.0.CO;2-Q.
Beal, M. F. (2003). Bioenergetic approaches for neuroprotection in Parkinson’s disease. Annals of Neurology, 53(Suppl 3), S39–S47. doi:10.1002/ana.10479.
Beal, M. F., & Ferrante, R. J. (2004). Experimental therapeutics in transgenic mouse models of Huntington’s disease. Nature Reviews. Neuroscience, 5, 373–384. doi:10.1038/nrn1386.
Benzi, G., & Ceci, A. (2001). Creatine as nutritional supplementation and medicinal product. Journal of Sports Medicine and Physical Fitness, 41, 1–10.
Bessman, S. P., & Geiger, P. J. (1981). Transport of energy in muscle: The phosphorylcreatine shuttle. Science, 211, 448–452. doi:10.1126/science.6450446.
Bindoff, L. A., Birch-Machin, M., Cartlidge, N. E., Parker, W. D., Jr., & Turnbull, D. M. (1989). Mitochondrial function in Parkinson’s disease. Lancet, 2, 49. doi:10.1016/S0140-6736(89)90291-2.
Boero, J., Qin, W., Cheng, J., Woolsey, T. A., Strauss, A. W., & Khuchua, Z. (2003). Restricted neuronal expression of ubiquitous mitochondrial creatine kinase: Changing patterns in development and with increased activity. Molecular and Cellular Biochemistry, 244, 69–76. doi:10.1023/A:1022409101641.
Bogdanov, M. B., Ferrante, R. J., Kuemmerle, S., Klivenyi, P., & Beal, M. F. (1998a). Increased vulnerability to 3-nitropropionic acid in an animal model of Huntington’s disease. Journal of Neurochemistry, 71, 2642–2644.
Bogdanov, M. B., Ramos, L. E., Xu, Z., & Beal, M. F. (1998b). Elevated “hydroxyl radical” generation in vivo in an animal model of amyotrophic lateral sclerosis. Journal of Neurochemistry, 71, 1321–1324.
Brewer, G. J., & Wallimann, T. W. (2000). Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. Journal of Neurochemistry, 74, 1968–1978. doi:10.1046/j.1471-4159.2000.0741968.x.
Brouillet, E., Jenkins, B. G., Hyman, B. T., Ferrante, R. J., Kowall, N. W., Srivastava, R., et al. (1993). Age-dependent vulnerability of the striatum to the mitochondrial toxin 3-nitropropionic acid. Journal of Neurochemistry, 60, 356–359. doi:10.1111/j.1471-4159.1993.tb05859.x.
Brouillet, E., Hantraye, P., Ferrante, R. J., Dolan, R., Leroy-Willig, A., Kowall, N. W., et al. (1995). Chronic mitochondrial energy impairment produces selective striatal degeneration and abnormal choreiform movements in primates. Proceedings of the National Academy of Sciences of the United States of America, 92, 7105–7109. doi:10.1073/pnas.92.15.7105.
Browne, S. E., Bowling, A. C., MacGarvey, U., Baik, M. J., Berger, S. C., Muqit, M. M., et al. (1997). Oxidative damage and metabolic dysfunction in Huntington’s disease: Selective vulnerability of the basal ganglia. Annals of Neurology, 41, 646–653. doi:10.1002/ana.410410514.
Browne, S. E., Ferrante, R. J., & Beal, M. F. (1999). Oxidative stress in Huntington’s disease. Brain Pathology, 9, 147–163.
Burklen, T. S., Schlattner, U., Homayouni, R., Gough, K., Rak, M., Szeghalmi, A., et al. (2006). The Creatine Kinase/Creatine Connection to Alzheimer’s Disease: CK-Inactivation, APP-CK Complexes and Focal Creatine Deposits. Journal of Biomedicine and Biotechnology, 2006, 35936. doi:10.1155/JBB/2006/35936.
Butterfield, D. A., & Lauderback, C. M. (2002). Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: Potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radical Biology and Medicine, 32, 1050–1060. doi:10.1016/S0891-5849(02)00794-3.
Candlish, E., La, C. J., & Unrau, A. M. (1969). The biosynthesis of 3-nitropropionic acid in creeping indigo (Indigofera spicata). Biochemistry, 8, 182–186. doi:10.1021/bi00829a026.
Carri, M. T., Ferri, A., Battistoni, A., Famhy, L., Gabbianelli, R., Poccia, F., et al. (1997). Expression of a Cu, Zn superoxide dismutase typical of familial amyotrophic lateral sclerosis induces mitochondrial alteration and increase of cytosolic Ca2+ concentration in transfected neuroblastoma SH-SY5Y cells. FEBS Letters, 414, 365–368. doi:10.1016/S0014-5793(97)01051-X.
Castegna, A., Aksenov, M., Thongboonkerd, V., Klein, J. B., Pierce, W. M., Booze, R., et al. (2002a). Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part II: Dihydropyrimidinase-related protein 2, alpha-enolase and heat shock cognate 71. Journal of Neurochemistry, 82, 1524–1532. doi:10.1046/j.1471-4159.2002.01103.x.
Castegna, A., Aksenov, M., Aksenova, M., Thongboonkerd, V., Klein, J. B., Pierce, W. M., et al. (2002b). Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part I: Creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radical Biology and Medicine, 33, 562–571. doi:10.1016/S0891-5849(02)00914-0.
Ceddia, R. B., & Sweeney, G. (2004). Creatine supplementation increases glucose oxidation and AMPK phosphorylation and reduces lactate production in L6 rat skeletal muscle cells. Journal of Physiology, 555, 409–421.
Csukly, K., Ascah, A., Matas, J., Gardiner, P. F., Fontaine, E., & Burelle, Y. (2006). Muscle denervation promotes opening of the permeability transition pore and increases the expression of cyclophilin D. Journal of Physiology, 574, 319–327. doi:10.1113/jphysiol.2006.109702.
Cui, L., Jeong, H., Borovecki, F., Parkhurst, C. N., Tanese, N., & Krainc, D. (2006). Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell, 127, 59–69. doi:10.1016/j.cell.2006.09.015.
David, S., Shoemaker, M., & Haley, B. E. (1998). Abnormal properties of creatine kinase in Alzheimer’s disease brain: Correlation of reduced enzyme activity and active site photolabeling with aberrant cytosol-membrane partitioning. Brain Research. Molecular Brain Research, 54, 276–287. doi:10.1016/S0169-328X(97)00343-4.
de la Monte, S. M., Luong, T., Neely, T. R., Robinson, D., & Wands, J. R. (2000). Mitochondrial DNA damage as a mechanism of cell loss in Alzheimer’s disease. Laboratory Investigation, 80, 1323–1335. doi:10.1038/labinvest.3780140.
Dedeoglu, A., Kubilus, J. K., Yang, L., Ferrante, K. L., Hersch, S. M., Beal, M. F., et al. (2003). Creatine therapy provides neuroprotection after onset of clinical symptoms in Huntington’s disease transgenic mice. Journal of Neurochemistry, 85, 1359–1367. doi:10.1046/j.1471-4159.2003.01706.x.
Di Lisa, F., & Bernardi, P. (2006). Mitochondria and ischemia-reperfusion injury of the heart: Fixing a hole. Cardiovascular Research, 70, 191–199. doi:10.1016/j.cardiores.2006.01.016.
Dolder, M., Walzel, O., Speer, U., Schlattner, T., & Wallimann, T. (2003). Inhibition of the mitochondrial transition by creatine kinase substrates. Requirement for microcompartmentation. Journal of Biological Chemistry, 278, 17760–17766. doi:10.1074/jbc.M208705200.
Eppenberger, H. M., Dawson, D. M., & Kaplan, N. O. (1967). The comparative enzymology of creatine kinases. I. Isolation and characterization from chicken and rabbit tissues. Journal of Biological Chemistry, 242, 204–209.
Ferrante, R. J., Andreassen, O. A., Jenkins, B. G., Dedeoglu, A., Kuemmerle, S., Kubilus, J. K., et al. (2000). Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. Journal of Neuroscience, 20, 4389–4397.
Gallant, M., Rak, M., Szeghalmi, A., Del Bigio, M. R., Westaway, D., Yang, J., et al. (2006). Focally elevated creatine detected in amyloid precursor protein (APP) transgenic mice and Alzheimer disease brain tissue. Journal of Biological Chemistry, 281, 5–8. doi:10.1074/jbc.C500244200.
Green, D. R., & Reed, J. C. (1998). Mitochondria and apoptosis. Science, 281, 1309–1312. doi:10.1126/science.281.5381.1309.
Groeneveld, G. J., Van Kan, H. J., Kalmijn, S., Veldink, J. H., Guchelaar, H. J., Wokke, J. H., et al. (2003). Riluzole serum concentrations in patients with ALS: Associations with side effects and symptoms. Neurology, 61, 1141–1143.
Grunewald, T., & Beal, M. F. (1999). Bioenergetics in Huntington’s disease. Annals of the New York Academy of Sciences, 893, 203–213. doi:10.1111/j.1749-6632.1999.tb07827.x.
Gu, M., Gash, M. T., Mann, V. M., Javoy-Agid, F., Cooper, J. M., & Schapira, A. H. (1996). Mitochondrial defect in Huntington’s disease caudate nucleus. Annals of Neurology, 39, 385–389. doi:10.1002/ana.410390317.
Gurney, M. E., Pu, H., Chiu, A. Y., Dal Canto, M. C., Polchow, C. Y., Alexander, D. D., et al. (1994). Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science, 264, 1772–1775. doi:10.1126/science.8209258.
Henshaw, R., Jenkins, B. G., Schulz, J. B., Ferrante, R. J., Kowall, N. W., Rosen, B. R., et al. (1994). Malonate produces striatal lesions by indirect NMDA receptor activation. Brain Research, 647, 161–166. doi:10.1016/0006-8993(94)91412-5.
Hensley, K., Butterfield, D. A., Mattson, M., Aksenova, M., Harris, M., Wu, J. F., et al. (1995). A model for beta-amyloid aggregation and neurotoxicity based on the free radical generating capacity of the peptide: Implications of “molecular shrapnel” for Alzheimer’s disease. Proceedings of the Western Pharmacology Society, 38, 113–120.
Hersch, S. M., Gevorkian, S., Marder, K., Moskowitz, C., Feigin, A., Cox, M., et al. (2006). Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2’dG. Neurology, 66, 250–252. doi:10.1212/01.wnl.0000194318.74946.b6.
Hervias, I., Beal, M. F., & Manfredi, G. (2006). Mitochondrial dysfunction and amyotrophic lateral sclerosis. Muscle and Nerve, 33, 598–608. doi:10.1002/mus.20489.
Hoyer, S. (2004). Causes and consequences of disturbances of cerebral glucose metabolism in sporadic Alzheimer disease: Therapeutic implications. Advances in Experimental Medicine and Biology, 541, 135–152.
Jacobus, W. E., & Lehninger, A. L. (1973). Creatine kinase of rat heart mitochondria. Coupling of creatine phosphorylation to electron transport. Journal of Biological Chemistry, 248, 4803–4810.
Jenkins, B. G., Koroshetz, W. J., Beal, M. F., & Rosen, B. R. (1993). Evidence for impairment of energy metabolism in vivo in Huntington’s disease using localized 1H NMR spectroscopy. Neurology, 43, 2689–2695.
Jost, C. R., Van Der Zee, C. E., In ‘t Zandt, H. J., Oerlemans, F., Verheij, M., Streijger, F., et al. (2002). Creatine kinase B-driven energy transfer in the brain is important for habituation and spatial learning behaviour, mossy fibre field size and determination of seizure susceptibility. European Journal of Neuroscience, 15, 1692–1706.
Juhn, M. S., & Tarnopolsky, M. (1998a). Oral creatine supplementation and athletic performance: A critical review. Clinical Journal of Sport Medicine, 8, 286–297.
Juhn, M. S., & Tarnopolsky, M. (1998b). Potential side effects of oral creatine supplementation: A critical review. Clinical Journal of Sport Medicine, 8, 298–304.
Klivenyi, P., Ferrante, R. J., Matthews, R. T., Bogdanov, M. B., Klein, A. M., Andreassen, O. A., et al. (1999). Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nature Medicine, 5, 347–350. doi:10.1038/6568.
Kokoszka, J. E., Waymire, K. G., Levy, S. E., Sligh, J. E., Cai, J., Jones, D. P., et al. (2004). The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature, 427, 461–465. doi:10.1038/nature02229.
Koroshetz, W. J., Jenkins, B. G., Rosen, B. R., & Beal, M. F. (1997). Energy metabolism defects in Huntington’s disease and effects of coenzyme Q10. Annals of Neurology, 41, 160–165. doi:10.1002/ana.410410206.
Krige, D., Carroll, M. T., Cooper, J. M., Marsden, C. D., & Schapira, A. H. (1992). Platelet mitochondrial function in Parkinson’s disease. The Royal Kings and Queens Parkinson Disease Research Group. Annals of Neurology, 32, 782–788. doi:10.1002/ana.410320612.
Li, X., Burklen, T., Yuan, X., Schlattner, U., Desiderio, D. M., Wallimann, T., et al. (2006). Stabilization of ubiquitous mitochondrial creatine kinase preprotein by APP family proteins. Molecular and Cellular Neurosciences, 31, 263–272. doi:10.1016/j.mcn.2005.09.015.
Ludolph, A. C., He, F., Spencer, P. S., Hammerstad, J., & Sabri, M. (1991). 3-Nitropropionic acid-exogenous animal neurotoxin and possible human striatal toxin. Canadian Journal of Neurological Sciences, 18, 492–498.
Ludolph, A. C., Seelig, M., Ludolph, A. G., Sabri, M. I., & Spencer, P. S. (1992). ATP deficits and neuronal degeneration induced by 3-nitropropionic acid. Annals of the New York Academy of Sciences, 648, 300–302. doi:10.1111/j.1749-6632.1992.tb24562.x.
Mahoney, D. J., Parise, G., & Tarnopolsky, M. A. (2002). Nutritional and exercise-based therapies in the treatment of mitochondrial disease. Current Opinion in Clinical Nutrition and Metabolic Care, 5, 619–629. doi:10.1097/00075197-200211000-00004.
Markesbery, W. R. (1997). Oxidative stress hypothesis in Alzheimer’s disease. Free Radical Biology and Medicine, 23, 134–147. doi:10.1016/S0891-5849(96)00629-6.
Matthews, R. T., Yang, L., Jenkins, B. G., Ferrante, R. J., Rosen, B. R., Kaddurah-Daouk, R., et al. (1998). Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington’s disease. Journal of Neuroscience, 18, 156–163.
Matthews, R. T., Ferrante, R. J., Klivenyi, P., Yang, L., Klein, A. M., Mueller, G., et al. (1999). Creatine and cyclocreatine attenuate MPTP neurotoxicity. Experimental Neurology, 157, 142–149. doi:10.1006/exnr.1999.7049.
Maurer, I., Zierz, S., & Moller, H. J. (2000). A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiology of Aging, 21, 455–462. doi:10.1016/S0197-4580(00)00112-3.
Mihic, S., MacDonald, J. R., McKenzie, S., & Tarnopolsky, M. A. (2000). Acute creatine loading increases fat-free mass, but does not affect blood pressure, plasma creatinine, or CK activity in men and women. Medicine and Science in Sports and Exercise, 32, 291–296. doi:10.1097/00005768-200002000-00007.
NINDS NET-PD Investigators. (2006). A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology, 66, 664–671. doi:10.1212/01.wnl.0000201252.57661.e1.
O’Gorman, E., Piendl, T., Muller, M., Brdiczka, D., & Wallimann, T. (1997a). Mitochondrial intermembrane inclusion bodies: The common denominator between human mitochondrial myopathies and creatine depletion, due to impairment of cellular energetics. Molecular and Cellular Biochemistry, 174, 283–289. doi:10.1023/A:1006881113149.
O’Gorman, E., Beutner, G., Dolder, M., Koretsky, A. P., Brdiczka, D., & Wallimann, T. (1997b). The role of creatine kinase in inhibition of mitochondrial permeability transition. FEBS Letters, 414, 253–257. doi:10.1016/S0014-5793(97)01045-4.
Onyango, I. G., & Khan, S. M. (2006). Oxidative stress, mitochondrial dysfunction, and stress signaling in Alzheimer’s disease. Current Alzheimer Research, 3, 339–349. doi:10.2174/156720506778249489.
Palfi, S., Ferrante, R. J., Brouillet, E., Beal, M. F., Dolan, R., Guyot, M. C., et al. (1996). Chronic 3-nitropropionic acid treatment in baboons replicates the cognitive and motor deficits of Huntington’s disease. Journal of Neuroscience, 16, 3019–3025.
Parker, W. D., Jr, Boyson, S. J., & Parks, J. K. (1989). Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Annals of Neurology, 26, 719–723. doi:10.1002/ana.410260606.
Parker, W. D., Jr. (1991). Cytochrome oxidase deficiency in Alzheimer’s disease. Annals of the New York Academy of Sciences, 640, 59–64.
Peng, T. I., & Greenamyre, J. T. (1998). Privileged access to mitochondria of calcium influx through N-methyl-d-aspartate receptors. Molecular Pharmacology, 53, 974–980.
Persky, A. M., & Brazeau, G. A. (2001). Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacological Reviews, 53, 161–176.
Pettegrew, J. W., Panchalingam, K., Klunk, W. E., McClure, R. J., & Muenz, L. R. (1994). Alterations of cerebral metabolism in probable Alzheimer’s disease: A preliminary study. Neurobiology of Aging, 15, 117–132. doi:10.1016/0197-4580(94)90152-X.
Phukan, J., Pender, N. P., & Hardiman, O. (2007). Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurology, 6, 994–1003. doi:10.1016/S1474-4422(07)70265-X.
Poortmans, J. R., Auquier, H., Renaut, V., Durussel, A., Saugy, M., & Brisson, G. R. (1997). Effect of short-term creatine supplementation on renal responses in men. European Journal of Applied Physiology and Occupational Physiology, 76, 566–567. doi:10.1007/s004210050291.
Poortmans, J. R., & Francaux, M. (2000). Adverse effects of creatine supplementation: Fact or fiction? Sports Medicine, 30, 155–170. doi:10.2165/00007256-200030030-00002.
Primeau, A. J., Adhihetty, P. J., & Hood, D. A. (2002). Apoptosis in heart and skeletal muscle. Canadian Journal of Applied Physiology, 27, 349–395.
Rae, C., Digney, A. L., McEwan, S. R., & Bates, T. C. (2003). Oral creatine monohydrate supplementation improves brain performance: A double-blind, placebo-controlled, cross-over trial. Proceedings. Biological Sciences, 270, 2147–2150. doi:10.1098/rspb.2003.2492.
Robinson, T. M., Sewell, D. A., Casey, A., Steenge, G., & Greenhaff, P. L. (2000). Dietary creatine supplementation does not affect some haematological indices, or indices of muscle damage and hepatic and renal function. British Journal of Sports Medicine, 34, 284–288. doi:10.1136/bjsm.34.4.284.
Ryu, H., & Ferrante, R. J. (2005). Emerging chemotherapeutic strategies for Huntington’s disease. Expert Opinion on Emerging Drugs, 10, 345–363. doi:10.1517/14728214.10.2.345.
Saks, V. A., Rosenshtraukh, L. V., Smirnov, V. N., & Chazov, E. I. (1978). Role of creatine phosphokinase in cellular function and metabolism. Canadian Journal of Physiology and Pharmacology, 56, 691–706.
Schapira, A. H., Cooper, J. M., Dexter, D., Clark, J. B., Jenner, P., & Marsden, C. D. (1990). Mitochondrial complex I deficiency in Parkinson’s disease. Journal of Neurochemistry, 54, 823–827. doi:10.1111/j.1471-4159.1990.tb02325.x.
Schlattner, U., Tokarska-Schlattner, M., & Wallimann, T. (2006). Mitochondrial creatine kinase in human health and disease. Biochimica et Biophysica Acta, 1762, 164–180.
Schulz, J. B., & Beal, M. F. (1995). Neuroprotective effects of free radical scavengers and energy repletion in animal models of neurodegenerative disease. Annals of the New York Academy of Sciences, 765, 100–110. doi:10.1111/j.1749-6632.1995.tb16565.x.
Selkoe, D. J. (1999). Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature, 399, A23–A31. doi:10.1038/19866.
Shefner, J. M., Cudkowicz, M. E., Schoenfeld, D., Conrad, T., Taft, J., Chilton, M., et al. (2004). A clinical trial of creatine in ALS. Neurology, 63, 1656–1661.
Simon, D. K., & Johns, D. R. (1999). Mitochondrial disorders: Clinical and genetic features. Annual Review of Medicine, 50, 111–127. doi:10.1146/annurev.med.50.1.111.
Small, D. H., & McLean, C. A. (1999). Alzheimer’s disease and the amyloid beta protein: What is the role of amyloid? Journal of Neurochemistry, 73, 443–449. doi:10.1046/j.1471-4159.1999.0730443.x.
Smith, C. D., Carney, J. M., Starke-Reed, P. E., Oliver, C. N., Stadtman, E. R., Floyd, R. A., et al. (1991). Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America, 88, 10540–10543. doi:10.1073/pnas.88.23.10540.
Sora, I., Richman, J., Santoro, G., Wei, H., Wang, Y., Vanderah, T., et al. (1994). The cloning and expression of a human creatine transporter. Biochemical and Biophysical Research Communications, 204, 419–427. doi:10.1006/bbrc.1994.2475.
Steeghs, K., Benders, A., Oerlemans, F., de, H. A., Heerschap, A., Ruitenbeek, W., et al. (1997). Altered Ca2+ responses in muscles with combined mitochondrial and cytosolic creatine kinase deficiencies. Cell, 89, 93–103. doi:10.1016/S0092-8674(00)80186-5.
Steenge, G. R., Lambourne, J., Casey, A., Macdonald, I. A., & Greenhaff, P. L. (1998). Stimulatory effect of insulin on creatine accumulation in human skeletal muscle. American Journal of Physiology, 275, E974–E979.
Stockler, S., Marescau, B., De Deyn, P. P., Trijbels, J. M., & Hanefeld, F. (1997). Guanidino compounds in guanidinoacetate methyltransferase deficiency, a new inborn error of creatine synthesis. Metabolism, 46, 1189–1193. doi:10.1016/S0026-0495(97)90215-8.
Stockler, S., & Hanefeld, F. (1997). Guanidinoacetate methyltransferase deficiency: A newly recognized inborn error of creatine biosynthesis. Wiener Klinische Wochenschrift, 109, 86–88.
Streijger, F., Oerlemans, F., Ellenbroek, B. A., Jost, C. R., Wieringa, B., & Van Der Zee, C. E. (2005). Structural and behavioural consequences of double deficiency for creatine kinases BCK and UbCKmit. Behavioural Brain Research, 157, 219–234. doi:10.1016/j.bbr.2004.07.002.
Tarnopolsky, M. A., & Beal, M. F. (2001). Potential for creatine and other therapies targeting cellular energy dysfunction in neurological disorders. Annals of Neurology, 49, 561–574. doi:10.1002/ana.1028.
Tarnopolsky, M. A., & Safdar, A. (2008). The potential benefits of creatine and conjugated linoleic acid as adjuncts to resistance training in older adults. Applied Physiology, Nutrition, and Metabolism, 33, 213–227. doi:10.1139/H07-142.
Thomas, B., & Beal, M. F. (2007). Parkinson’s disease. Human Molecular Genetics, 16(Spec no. 2), R183–R194.
Valla, J., Berndt, J. D., & Gonzalez-Lima, F. (2001). Energy hypometabolism in posterior cingulate cortex of Alzheimer’s patients: Superficial laminar cytochrome oxidase associated with disease duration. Journal of Neuroscience, 21, 4923–4930.
van der Knaap, M. S., Verhoeven, N. M., Maaswinkel-Mooij, P., Pouwels, P. J., Onkenhout, W., Peeters, E. A., et al. (2000). Mental retardation and behavioral problems as presenting signs of a creatine synthesis defect. Annals of Neurology, 47, 540–543. doi:10.1002/1531-8249(200004)47:4<540::AID-ANA23>3.0.CO;2-K.
Wallimann, T., Wyss, M., Brdiczka, D., Nicolay, K., & Eppenberger, H. M. (1992). Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochemical Journal, 281(Pt 1), 21–40.
Wallimann, T., & Hemmer, W. (1994). Creatine kinase in non-muscle tissues and cells. Molecular and Cellular Biochemistry, 133–134, 193–220. doi:10.1007/BF01267955.
Watanabe, A., Kato, N., & Kato, T. (2002). Effects of creatine on mental fatigue and cerebral hemoglobin oxygenation. Neuroscience Research, 42, 279–285. doi:10.1016/S0168-0102(02)00007-X.
Weydt, P., Pineda, V. V., Torrence, A. E., Libby, R. T., Satterfield, T. F., Lazarowski, E. R., et al. (2006). Thermoregulatory and metabolic defects in Huntington’s disease transgenic mice implicate PGC-1alpha in Huntington’s disease neurodegeneration. Cell Metabolism, 4, 349–362. doi:10.1016/j.cmet.2006.10.004.
Wong, P. C., Pardo, C. A., Borchelt, D. R., Lee, M. K., Copeland, N. G., Jenkins, N. A., et al. (1995). An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron, 14, 1105–1116. doi:10.1016/0896-6273(95)90259-7.
Wyss, M., & Schulze, A. (2002). Health implications of creatine: Can oral creatine supplementation protect against neurological and atherosclerotic disease? Neuroscience, 112, 243–260. doi:10.1016/S0306-4522(02)00088-X.
Zong, H., Ren, J. M., Young, L. H., Pypaert, M., Mu, J., Birnbaum, M. J., et al. (2002). AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proceedings of the National Academy of Sciences of the United States of America, 99, 15983–15987. doi:10.1073/pnas.252625599.
Acknowledgements
The administrative assistance of Greta Strong, the editorial advice provided by Anna-Maria Joseph and the generous contribution of portions of the mitochondrial illustrations provided by Dr. David Hood's laboratory (York University, Toronto, ON, Canada) during the preparation of this manuscript are gratefully acknowledged. This work was supported by the NINDS, NIA, HDSA, and the Department of Defense. The authors have attempted to include all relevant topics/articles but realize that certain interesting aspects may have been excluded due to space constraints and we apologize for the inability to include these areas.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adhihetty, P.J., Beal, M.F. Creatine and Its Potential Therapeutic Value for Targeting Cellular Energy Impairment in Neurodegenerative Diseases. Neuromol Med 10, 275–290 (2008). https://doi.org/10.1007/s12017-008-8053-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-008-8053-y