Abstract
Background
Intracerebral hemorrhage (ICH) expansion is common during the first 24 h after onset, but the pattern and pace of hyperacute hemorrhage growth have not been described because serial imaging is typically performed over the course of hours and days, not minutes. The purpose of this study is to elucidate the spatial and temporal characteristics of hyperacute hemorrhage expansion within minutes of ICH onset.
Methods
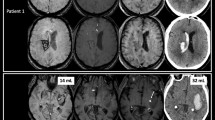
An 86-year-old man with probable cerebral amyloid angiopathy developed an ICH while in the MRI scanner. Hyperacute hemorrhage growth was captured at three time points over a 14-min interval of MRI data acquisition and at fourth time point with CT 22 h later. MRI and CT datasets were spatially coregistered, and three-dimensional models of ICH expansion were generated.
Results
Longitudinal analysis revealed that the spatial pattern of ICH growth was asymmetric at each time point. Maximal expansion occurred along the anterior–posterior plane during the first 4 min but along the superior–inferior plane during the next 10 min. The temporal pace of ICH expansion was also non-uniform, as growth along the anterior–posterior plane outpaced medial–lateral growth during the first 4 min (2.8 vs. 2.5 cm), but medial–lateral growth outpaced anterior–posterior growth over the next 10 min (1.0 vs. 0.2 cm).
Conclusions
We provide evidence for asymmetric, non-uniform expansion of a hyperacute hemorrhage. These serial imaging observations suggest that hemorrhage expansion may be caused by local cascades of secondary vessel rupture as opposed to ongoing bleeding from a single ruptured vessel.



Similar content being viewed by others
References
Kazui S, Naritomi H, Yamamoto H, Sawada T, Yamaguchi T. Enlargement of spontaneous intracerebral hemorrhage: incidence and time course. Stroke. 1996;27:1783–7.
Brott T, Broderick J, Kothari R, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5.
Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Tanaka R. Multivariate analysis of predictors of hematoma enlargement in spontaneous intracerebral hemorrhage. Stroke. 1998;29:1160–6.
Fisher CM. Pathological observations in hypertensive cerebral hemorrhage. J Neuropathol Exp Neurol. 1971;30:536–50.
Jeong D, Jhaveri MD, Prabhakaran S. Magnetic resonance imaging characteristics at onset of spontaneous intracerebral hemorrhage. Arch Neurol. 2011;68:826–7.
Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–9.
Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–5.
Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19.
Wells WM 3rd, Viola P, Atsumi H, Nakajima S, Kikinis R. Multi-modal volume registration by maximization of mutual information. Med Image Anal. 1996;1:35–51.
Wang R and Wedeen VJ. TrackVis, Version 5.2, Athinoula A. Martinos Center for Biomedical Imaging Massachusetts General Hospital, Charlestown. http://www.trackvis.org, 2011.
Fisher CM. Hypertensive cerebral hemorrhage. Demonstration of the source of bleeding. J Neuropathol Exp Neurol. 2003;62:104–7.
Greenberg CH, Frosch MP, and Greenberg SM. Modeling the growth of microbleeds into macrobleeds (P485). Stroke: Abstracts from the 2010 International Stroke Conference. 2010;41:e376.
Rosand J, Muzikansky A, Kumar A, et al. Spatial clustering of hemorrhages in probable cerebral amyloid angiopathy. Ann Neurol. 2005;58:459–62.
Brouwers HB, Biffi A, Ayres AM, et al. Apolipoprotein e genotype predicts hematoma expansion in lobar intracerebral hemorrhage. Stroke. 2012;43:1490–5.
Greenberg SM, Vonsattel JP, Segal AZ, et al. Association of apolipoprotein E epsilon2 and vasculopathy in cerebral amyloid angiopathy. Neurology. 1998;50:961–5.
Greenberg SM, Nandigam RN, Delgado P, et al. Microbleeds versus macrobleeds: evidence for distinct entities. Stroke. 2009;40:2382–6.
Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–37.
Anderson CS, Huang Y, Wang JG, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol. 2008;7:391–9.
ATACH Investigators. Antihypertensive treatment of acute cerebral hemorrhage. Crit Care Med. 2010;38:637–48.
Acknowledgments
This study was supported by the NIH (R25NS065743).
Conflict of interest
The authors report no conflicts of interest or pertinent financial disclosures.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Video Hyperacute hemorrhage expansion. Hemorrhage expansion is shown at each sequential time point of MRI and CT data acquisition, with time displayed in hour:minute format and with the corresponding hemorrhage volume displayed at each time point. The first part of the video displays hemorrhage expansion from a superior view, with 3-dimensional renderings of the hemorrhage (red) superimposed on an axial T1 pre-contrast image at the level of the cerebral microbleed seen on the gradient-recalled echo sequence. In the second part of the video, hemorrhage expansion is displayed from a posterior view, with 3-dimensional renderings of the hemorrhage superimposed on the same T1 pre-contrast axial image, as well as a T1 pre-contrast coronal image at the level of the thalamus. Of note, all images are displayed in non-radiological orientation, with the left side of the image (L) on the left side of the screen, and the right side of the image (R) on the right side of the screen. (MOV 2572 kb)
Rights and permissions
About this article
Cite this article
Edlow, B.L., Bove, R.M., Viswanathan, A. et al. The Pattern and Pace of Hyperacute Hemorrhage Expansion. Neurocrit Care 17, 250–254 (2012). https://doi.org/10.1007/s12028-012-9738-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-012-9738-5