Abstract
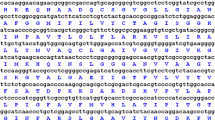
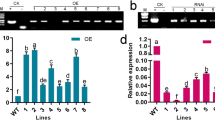
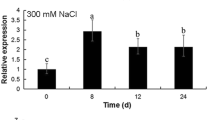
An aquaporin cDNA BjPIP1 isolated from heavy-metal accumulator Indian mustard (Brassica juncea L.) encodes a 286-residue protein. The deduced amino acid sequence of BjPIP1 with six putative transmembrane domains showed highest identity (85–99%) to PIP1 subfamily members. Semi-quantitative RT-PCR analysis revealed that BjPIP1 transcripts were more abundantly expressed in roots compared to aerial parts of Indian mustard. However, the expression of BjPIP1 in leaves was up-regulated by drought, salt, low temperature, and heavy metal stress, suggesting that BjPIP1 was involved in resistance to abiotic stresses. BjPIP1 under the control of 35S promoter was introduced into tobacco mediated with Agrobacterium tumefaciens, the transgenic tobacco exhibited a lower water loss rate, a decreased transpiration rate, and stomatal conductance compared to the wild-type plants under osmotic stress, indicating that BjPIP1 might enhance plant drought resistance by decreasing transpiration via reducing stomatal conductance. Furthermore, overexpression of BjPIP1 in tobacco enhanced Cd resistance of root growth, and lowered transpiration rate and stomatal conductance upon Cd exposure, suggesting that BjPIP1 might increase heavy-metal resistance by maintaining reasonable water status in tobacco. Moreover, the BjPIP1-overexpressing plants showed higher activities of antioxidative enzymes, and lower level of electrolyte leakage and malondialdehyde content under Cd stress, indicating BjPIP1 might enhance the antioxidative activity and membrane integrity in transgenic plants. Taken together, these results suggested that BjPIP1 might improve plant heavy-metal resistance through alleviating water deficit and oxidative damage induced by metal ions.












Similar content being viewed by others
Abbreviations
- CAT:
-
Catalase
- EC:
-
Electrical conductivity
- MDA:
-
Malondialchehyche
- PEG:
-
Polyethylene glycol
- POD:
-
Peroxidase
- SOD:
-
Superoxide dismutase
References
Blumwald, E. (2000). Sodium transport and salt tolerance in plants. Current Opinion in Cell Biology, 12, 431–434. doi:10.1016/S0955-0674(00)00112-5.
Zhu, J. K. (2001). Cell signal under salt, water and cold stresses. Current Opinion in Plant Biology, 4, 401–406. doi:10.1016/S1369-5266(00)00192-8.
Walz, T., Typke, D., Smith, B. L., Agre, P., & Engel, A. (1995). Projection map of aquaporin-1 determined by electron crystallography. Nature Structural Biology, 3, 730–732. doi:10.1038/nsb0995-730.
Johansson, I., Karlsson, M., Johanson, U., Larsson, C., & Kjellbom, P. (2000). The role of aquaporins in cellular and whole plant water balance. Biochimica et Biophysica Acta, 1465, 324–342. doi:10.1016/S0005-2736(00)00147-4.
Johanson, U., Karlsson, M., Johansson, I., Gustavsson, S., Sjövall, S., Fraysse, L., et al. (2001). The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiology, 126, 1358–1369. doi:10.1104/pp.126.4.1358.
Chaumont, F., Barrieu, F., Wojcik, E., Chrispeels, M. J., & Jung, R. (2001). Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiology, 125, 1206–1215. doi:10.1104/pp.125.3.1206.
Sakurai, J., Ishikawa, F., Yamaguchi, T., Uemura, M., & Maeshima, M. (2005). Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant and Cell Physiology, 46, 1568–1577. doi:10.1093/pcp/pci172.
Rizhsky, L., Liang, H., Shuman, J., Shulaev, V., Davletova, S., & Mittler, R. (2004). When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiology, 134, 1683–1696. doi:10.1104/pp.103.033431.
Oono, Y., Seki, M., Nanjo, T., Narusaka, M., Fujita, M., Satoh, R., et al. (2003). Monitoring expression profiles of Arabidopsis gene expression during rehydration process after dehydration using ca. 7000 full-length cDNA microarray. Plant Journal, 34, 868–887. doi:10.1046/j.1365-313X.2003.01774.x.
Oono, Y., Seki, M., Satou, M., Iida, K., Akiyama, K., Sakurai, T., et al. (2006). Monitoring expression profiles of Arabidopsis genes during cold acclimation and deacclimation using DNA microarrays. Functional and Integrative Genomics, 6, 212–234. doi:10.1007/s10142-005-0014-z.
Houde, M., Belcaid, M., Ouellet, F., Danyluk, J., Monroy, A. F., Dryanova, A., et al. (2006). Wheat EST resources for functional genomics of abiotic stress. BMC Genomics, 7, 149. doi:10.1186/1471-2164-7-149.
Cramer, G. R., Ergül, A., Grimplet, J., Tillett, R. L., Tattersall, E. A. R., Bohlman, M. C., et al. (2007). Water and salinity stress in grapevines: early and late changes in transcript and metabolite profiles. Functional and Integrative Genomics, 7, 111–134. doi:10.1007/s10142-006-0039-y.
Martre, P., Morillon, R., Barrieu, F., North, G. B., Nobel, S. P., & Chrispeels, M. J. (2002). Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiology, 130, 2101–2110. doi:10.1104/pp.009019.
Siefritz, F., Tyree, M. T., Lovisolo, C., Schubert, A., & Kaldenhoff, R. (2002). PIP1 plasma membrane aquaporins in tobacco: From cellular effects to function in plants. Plant Cell, 14, 869–876. doi:10.1105/tpc.000901.
Javot, H., Lauvergeat, V., Santoni, V., Martin-Laurent, F., Güçlü, J., Vinh, J., et al. (2003). Role of a single aquaporin isoform in root water uptake. The Plant Cell, 15, 509–522. doi:10.1105/tpc.008888.
Aharon, R., Shahak, Y., Wininger, S., Bendov, R., Kapulnik, Y., & Galili, G. (2003). Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but to under drought or salt stress. The Plant Cell, 15, 439–447. doi:10.1105/tpc.009225.
Clemens, S. (2006). Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie, 88, 1707–1719. doi:10.1016/j.biochi.2006.07.003.
Zenk, M. H. (1996). Heavy metal detoxification in higher plants: A review. Gene, 179, 21–23. doi:10.1016/S0378-1119(96)00422-2.
Sanita di Toppi, L., & Gabbrielli, R. (1999). Response to cadmium in higher plants. Environmental and Experimental Botany, 41, 105–130. doi:10.1016/S0098-8472(98)00058-6.
Prasad, M. N. V. (1997). Trace metals. In M. N. V. Prasad (Ed.), Plant ecophysiology (pp. 207–249). New York: Wiley.
Barcelo, J., Poschenrieder, C., Andreu, I., & Gunse, B. (1986). Cadmium-induced decrease of water stress resistance in bush bean plants (Phaseolus vulgaris L. cv. Contender) I. Effects of Cd on water potential, relative water content and cell wall elasticity. Journal of Plant Physiology, 125, 17–25.
Pandey, N., & Sharma, C. P. (2002). Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Science, 163, 753–758. doi:10.1016/S0168-9452(02)00210-8.
Maggio, A., & Joly, R. J. (1995). Effects of mercuric chloride on the hydraulic conductivity of tomato root systems (evidence for a channel-mediated water pathway). Plant Physiology, 109, 331–335.
Tazawa, M., Ohkuma, E., Shibasaka, M., & Nakashima, S. (1997). Mercurial sensitive water transport in barley roots. Journal of Plant Research, 110, 435–442. doi:10.1007/BF02506803.
Agre, P., Bonhivers, M., & Borgnia, M. J. (1998). The aquaporins, blueprints for cellular plumbing systems. The Journal of Biological Chemistry, 273, 14659–14662. doi:10.1074/jbc.273.24.14659.
Reiter, R. S., Young, R. M., & Scolnik, P. A. (1992). Genetic linkage of the Arabidopsis genome: Methods for mapping with recombinant inbreds and random amplified polymorphic DNAs (RAPDs). In C. Konc, N. H. Chua, & J. Schell (Eds.), Methods in Arabidopsis research (pp. 170–190). Singapore: World Scientific.
Kramer, G. F., Norman, H. A., Krizek, D. T., & Mirecki, R. M. (1991). Influence of UV-B radiation on polyamines, lipid peroxidation and membrane lipids in cucumber. Phytochemistry, 30, 2101–2108. doi:10.1016/0031-9422(91)83595-C.
Alexieva, V., Sergiev, I., Mapelli, S., & Karanov, E. (2001). The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant, Cell and Environment, 24, 1337–1344. doi:10.1046/j.1365-3040.2001.00778.x.
Beauchamp, C., & Fridovich, I. (1971). Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry, 44, 276–287. doi:10.1016/0003-2697(71)90370-8.
Cakmak, I., & Horst, W. J. (1991). Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase and peroxidase activities in root tips of soybean (Glycine max). Plant Physiology, 83, 463–468. doi:10.1111/j.1399-3054.1991.tb00121.x.
Nickel, R. S., & Cunningham, B. A. (1969). Improved peroxidase assay method using Ieuco 2, 3, 6-trichlcroindophenol and application to comparative measurements of peroxidase catalysis. Analytical Biochemistry, 27, 292–299. doi:10.1016/0003-2697(69)90035-9.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. doi:10.1016/0003-2697(76)90527-3.
Ruiter, R. K., van Eldik, G. J., van Herpen, M. M. A., Schrauwen, J. A. M., & Wullems, G. J. (1997). Expression in anthers of two genes encoding Brassica oleracea transmembrane channel proteins. Plant Molecular Biology, 34, 163–167. doi:10.1023/A:1005828325425.
Cui, X. H., Hao, F. S., Chen, H., Cai, J. H., Chen, J., & Wang, X. C. (2005). Isolation and expression of an aquaporin-like gene VfPIP1 in Vicia faba. Progress in Natural Science, 15, 496–501. doi:10.1080/10020070512331342460.
Lian, H. L., Yu, X., Ye, Q., Ding, X., Kitagawa, Y., Kwak, S. S., et al. (2004). The role of aquaporin RWC3 in drought avoidance in rice. Plant and Cell Physiology, 45, 481–489. doi:10.1093/pcp/pch058.
Thomashow, M. F. (1999). Plant cold acclimation, freezing tolerance genes and regulatory mechanisms. Plant Molecular Biology, 50, 571–599. doi:10.1146/annurev.arplant.50.1.571.
Otto, B., & Kaldenhoff, R. (2000). Cell-specific expression of the mercury insensitive plasma-membrane aquaporin NtAQP1 from Nicotiana tabacum. Planta, 211, 167–172. doi:10.1007/s004250000275.
Alexandersson, E., Fraysee, L., Sjovall-Larsen, S., Gustavsson, S., Feller, M., Karlsson, M., et al. (2005). Whole gene family expression and drought stress regulation of aquaporins. Plant Molecular Biology, 59, 469–484. doi:10.1007/s11103-005-0352-1.
Guo, L., Wang, Z. Y., Lin, H., Cui, W. E., Chen, J., Liu, M., et al. (2006). Expression and functional analysis of the rice plasma membrane intrinsic protein gene family. Cell Research, 16, 277–286. doi:10.1038/sj.cr.7310035.
Secchi, F., Lovisolo, C., Uehlein, N., Kaldenhoff, R., & Schubert, A. (2007). Isolation and functional characterization of three aquaporins from olive (Olea europaea L.). Planta, 225, 381–392. doi:10.1007/s00425-006-0365-2.
Jang, J. Y., Kim, D. G., Kim, Y. O., Kim, J. S., & Kang, H. (2004). An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Molecular Biology, 54, 713–725. doi:10.1023/B:PLAN.0000040900.61345.a6.
Aroca, R., Amodeo, G., Fernandez-Illescas, S., Herman, E. M., Chaumont, F., & Chrispeels, M. J. (2005). The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiology, 137, 341–353. doi:10.1104/pp.104.051045.
Zhu, C., Schraut, D., Hartung, W., & Schaffner, A. R. (2005). Differential responses of maize MIP genes to salt stress and ABA. Journal of Experimental Botany, 56, 2971–2981. doi:10.1093/jxb/eri294.
Yu, Q. J., Hu, Y. L., Li, J. F., Wu, Q., & Lin, Z. P. (2005). Sense and antisense expression of plasma membrane aquaporin BnPIP1 from Brassica napus in tobacco and its effects on plant drought resistance. Plant Science, 169, 647–656. doi:10.1016/j.plantsci.2005.04.013.
Schurr, U., Gollan, T., & Schulze, E. D. (1992). Stomatal response to drying soil in relation to changes in the xylem sap composition of Helianthus annuus. II. Stomatal sensitivity to abscisic acid imported from the xylem sap. Plant, Cell and Environment, 15, 561–567. doi:10.1111/j.1365-3040.1992.tb01489.x.
Siddique, M. R. B., Hamid, A., & Islam, M. S. (1999). Drought stress effects on photosynthetic rate and leaf gas exchange of wheat. Botonical Bulletin of Academia Sinica, 40, 141–145.
Hanba, Y. T., Shibasaka, M., Hayashi, Y., Hayakawa, T., Kasamo, K., Terashima, I., et al. (2004). Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 assimilation in the leaves of transgenic rice plants. Plant and Cell Physiology, 45, 521–529. doi:10.1093/pcp/pch070.
Comparot, S., Morilon, R., & Badot, P. M. (2000). Water permeability and revolving movement in Phaseolus vulgaris L. Plant and Cell Physiology, 41, 114–118.
Katsuhara, M., Koshio, K., Shibasaka, M., Hayashi, Y., Hayakawa, T., & Kasamo, K. (2003). Overexpression of a barley aquaporin increased the shoot/root ratio and raised salt sensitivity in transgenic rice plants. Plant and Cell Physiology, 44, 1378–1383. doi:10.1093/pcp/pcg167.
Uehlein, N., Lovisolo, C., Siefritz, F., & Kaldenhoff, R. (2003). The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature, 425, 734–737. doi:10.1038/nature02027.
Costa, G., & Spitz, E. (1997). Influence of cadmium on soluble carbohydrates, free amino acids, proline content of in vitro cultured Lupinus albus. Plant Science, 128, 131–140. doi:10.1016/S0168-9452(97)00148-9.
Rauser, W. E., & Dumbroff, E. B. (1981). Effect of excess cobalt, nickel and zinc on water relations of Phaseolus vulgaris. Environmental and Experimental Botany, 21, 235–249. doi:10.1016/0098-8472(81)90032-0.
Alia, P., & Pardha Saradhi, P. (1991). Proline accumulation under heavy metal stress. Journal of Plant Physiology, 138, 554–558.
Alonso, A., Queiroz, C. S., & Magalhaes, A. C. (1997). Chilling stress leads to increased cell membrane rigidity in roots of coffee (Coffea arabica L) seedlings. Biochimica et Biophysica Acta, 1323, 75–84. doi:10.1016/S0005-2736(96)00177-0.
Dixit, V., Pandey, V., & Shyam, R. (2001). Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L cv. Azad). Journal of Experimental Botany, 52, 1101–1109. doi:10.1093/jexbot/52.358.1101.
Hachez, C., Zelazny, E., & Chaumont, F. (2006). Modulating the expression of aquaporin genes in planta: A key to understand their physiological functions? Biochimica et Biophysica Acta, 1758, 1142–1156. doi:10.1016/j.bbamem.2006.02.017.
Bienert, G. P., Schjoerring, J. K., & Jahn, T. P. (2006). Membrane transport of hydrogen peroxide. Biochimica et Biophysica Acta, 1758, 994–1003. doi:10.1016/j.bbamem.2006.02.015.
Acknowledgments
This research was supported by the National High Technology Planning Program of China (Grant nos. 2007AA021404 and 2006AA10Z407) and the Undergraduate Innovative Experiment Program of China University of Mining & Technology (Beijing) (Grant no. 0821).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, Y., Wang, Z., Chai, T. et al. Indian Mustard Aquaporin Improves Drought and Heavy-metal Resistance in Tobacco. Mol Biotechnol 40, 280–292 (2008). https://doi.org/10.1007/s12033-008-9084-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-008-9084-1