Abstract
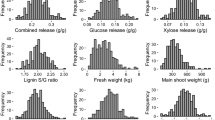
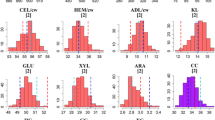
Short rotation coppice (SRC) willows (Salix spp.) are fast-growing woody plants which can achieve high biomass yields over short growth cycles with low agrochemical inputs. Biomass from SRC willow is already used for heat and power, but its potential as a source of lignocellulose for liquid transport biofuels has still to be assessed. In bioethanol production from lignocellulose, enzymatic saccharification is used as an approach to release glucose from cellulose in the plant cell walls. In this study, 138 genotypes of a willow mapping population were used to examine variation in enzymatic glucose release from stem biomass to study relationships between this trait and biomass yield traits and to identify quantitative trait loci (QTL) associated with enzymatic saccharification yield. Significant natural variation was found in glucose yields from willow stem biomass. This trait was independent of biomass yield traits. Four enzyme-derived glucose QTL were mapped onto chromosomes V, X, XI, and XVI, indicating that enzymatic saccharification yields are under significant genetic influence. Our results show that SRC willow has strong potential as a source of bioethanol and that there may be opportunities to improve the breeding programs for willows for increasing enzymatic saccharification yields and biofuel production.





Similar content being viewed by others
References
Meyers RJ (2008) Renewable fuel standard for 2009, 73(226). Federal Register, Issued Pursuant to Section 211(o) of the Clean Air Act, United States, November 14
Directive of the European Parliament and of the Council (2008) Promotion of the use of energy from renewable sources. European Union, Brussels
Secretary of State for Energy and Climate Change (2009) The UK renewable energy strategy. HM Government, London
Gomez LD, Steele-King CG, McQueen-Mason SJ (2008) Sustainable liquid biofuels from biomass: the writing’s on the walls. New Phytol 178(3):473–485
Karp A, Shield I (2008) Bioenergy from plants and the sustainable yield challenge. New Phytol 179(1):15–32
Rubin EM (2008) Genomics of cellulosic biofuels. Nature 454(7206):841–845
Ragauskas AJ et al (2006) The path forward for biofuels and biomaterials. Science 311(5760):484–489
Tilman D et al (2009) Beneficial biofuels-the food, energy, and environment trilemma. Science 325(5938):270–271
Searchinger T et al (2008) Use of US croplands for biofuels increases greenhouse gases through emissions from land-use change. Science 319(5867):1238–1240
Himmel ME (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production (vol 315, pg 804, 2007). Science 316(5827):982–982
Yang B, Wyman CE (2008) Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofpr 2(1):26–40
Armstrong A, Johns C, Tubby I (1999) Effects of spacing and cutting cycle on the yield of poplar grown as an energy crop. Biomass Bioenerg 17(4):305–314
Mitchell CP, Stevens EA, Watters MP (1999) Short-rotation forestry - operations, productivity and costs based on experience gained in the UK. For Ecol Manag 121(1-2):123–136
Bioenergy Scheme (2009) Best Practice Manual for SRC Willow. Bioenergy Scheme Department of Agriculture, Fisheries and Food, Dublin
Adegbidi HG et al (2001) Biomass and nutrient removal by willow clones in experimental bioenergy plantations in New York State. Biomass Bioenerg 20(6):399–411
Argus GW (1997) Infrageneric classification of Salix (Salicaceae) in the new world. Syst Bot Monogr 52:1–121
Trybush S, Jahodova S, Macalpine W, Karp A (2008) A genetic study of a Salix germplasm resource reveals new insights into relationships among subgenera, sections and species. BioEnergy Res 1(1):67–79
Cochard H, Casella E, Mencuccini M (2007) Xylem vulnerability to cavitation varies among poplar and willow clones and correlates with yield. Tree Physiol 27(12):1761–1767
Gullberg U (1993) Towards making willows pilot species for coppicing production. Forestry Chron 69(6):721–726
Hanley SJ (2003) Improving willow breeding efficiency. Thesis, University of Bristol
Larsson S (1998) Genetic improvement of willow for short-rotation coppice. Biomass Bioenerg 15(1):23–26
Robinson KM, Karp A, Taylor G (2004) Defining leaf traits linked to yield in short-rotation coppice Salix. Biomass Bioenerg 26(5):417–431
Ronnberg-Wastljung AC (2001) Genetic structure of growth and phenological traits in Salix viminalis. Can J Forest Res 31(2):276–282
Tharakan PJ, Volk TA, Nowak CA, Abrahamson LP (2005) Morphological traits of 30 willow clones and their relationship to biomass production. Can J Forest Res 35(2):421–431
Serapiglia MJ, Cameron KD, Stipanovic AJ, Smart LB (2008) High-resolution thermogravimetric analysis for rapid characterization of biomass composition and selection of shrub willow varieties. Appl Biochem Biotechnol 145(1–3):3–11
Rae AM et al (2008) QTL for yield in bioenergy Populus: identifying GxE interactions from growth at three contrasting sites. Tree Genet Genomes 4(1):97–112
Rae AM et al (2009) Five QTL hotspots for yield in short rotation coppice bioenergy poplar: the poplar biomass loci. BMC Plant Biol 9:23
Tsarouhas V, Gullberg U, Lagercrantz U (2003) Mapping of quantitative trait loci controlling timing of bud flush in Salix. Hereditas 138(3):172–178
Weih M, Ronnberg-Wastljung AC, Glynn C (2006) Genetic basis of phenotypic correlations among growth traits in hybrid willow (Salix dasyclados x S-viminalis) grown under two water regimes. New Phytol 170(3):467–477
Tsarouhas V, Gullberg U, Lagercrantz U (2004) Mapping of quantitative trait loci (QTLs) affecting autumn freezing resistance and phenology in Salix. Theor Appl Genet 108(7):1335–1342
Ronnberg-Wastljung AC, Glynn C, Weih M (2005) QTL analyses of drought tolerance and growth for a Salix dasyclados x Salix viminalis hybrid in contrasting water regimes. Theor Appl Genet 110(3):537–549
Hanley SJ, Mallott MD, Karp A (2006) Alignment of a Salix linkage map to the Populus genomic sequence reveals macrosynteny between willow and poplar genomes. Tree Genet Genomes 3(1):35–48
Christian DG, Riche AB, Yates NE (2008) Growth, yield and mineral content of Miscanthus x giganteus grown as a biofuel for 14 successive harvests. Ind Crops Prod 28(3):320–327
Selig M, Weiss N, Ji Y (2008) Enzymatic saccharification of lignocellulosic biomass. National Renewable Energy Laboratory, Golden
Mosier N et al (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96(6):673–686
Patterson HD, Thompson R (1971) Recovery of inter-block information when block sizes are unequal. Biometrika 58(3):545–554
GenStat® (2008) © Lawes Agricultural Trust (Rothamsted Research), 11th edn. VSN International, London
Maliepaard C, Van Ooijen JW (1994) QTL mapping in a full-sib family of an outcrossing species. Biometrics in plant breeding: applications of molecular markers. CPRO-DLO, Wageningen, pp 140–146
Lander ES, Botstein D (1989) Mapping Mendelian factors underlying quantitative traits using Rflp linkage maps. Genetics 121(1):185–199
Van Ooijen JW, Boer MP, Jansen RC, Maliepaard C (2002) MapQTL® 4.0. Plant Research International, Wageningen
Lindegaard KN, Parfitt RI, Doonaldson G, Hunter T, Dawson WM, Forbes EGA et al (2001) Comparative trials of elite Swedish and UK biomass willow varieties. Aspects Appl Biol 65:183–192
Alfenore S et al (2004) Aeration strategy: a need for very high ethanol performance in Saccharomyces cerevisiae fed-batch process. Appl Microbiol Biotechnol 63(5):537–542
Adler A, Verwijst T, Aronsson P (2005) Estimation and relevance of bark proportion in a willow stand. Biomass Bioenergy 29(2):102–113
Klasnja B, Kopitovic S, Orlovic S (2002) Wood and bark of some poplar and willow clones as fuelwood. Biomass Bioenergy 23(6):427–432
Larsson S et al (1999) The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb Technol 24(3–4):151–159
Sassner P, Galbe M, Zacchi G (2005) Steam pretreatment of Salix with and without SO2 impregnation for production of bioethanol. Appl Biochem Biotechnol 121(1–3):1101–1117
Sassner P, Martensson CG, Galbe M, Zacchi G (2008) Steam pretreatment of H2SO4-impregnated Salix for the production of bioethanol. Bioresour Technol 99(1):137–145
Acknowledgements
The authors gratefully acknowledge funding support from the Rothamsted BBSRC Bioenergy and Climate Change Institute Strategic Programme Grant and from the Porter Alliance (http://www.porteralliance.org.uk/) for a studentship awarded to Nick Brereton. Dr. Frederic Pitre was supported through a FQRNT postdoctoral research fellowship from the Government of Quebec, Canada. Many thanks are extended to staff of Fenswood Farm, Long Ashton, Bristol for their fast and thorough harvesting of the willow samples and to Dr. Ian Shield and Mr. William Macalpine at Rothamsted for their excellent advice and support for this work. We would also like to thank Stephen Powers for his assistance with statistical analysis. Rothamsted Research is an Institute of the Biotechnology and Biological Sciences Research Council (BBSRC) of the UK.
Author information
Authors and Affiliations
Corresponding author
Additional information
Imperial College London and Rothamsted Research are members of the Porter Alliance (http://www.porteralliance.org.uk/).
Rights and permissions
About this article
Cite this article
Brereton, N.J.B., Pitre, F.E., Hanley, S.J. et al. QTL Mapping of Enzymatic Saccharification in Short Rotation Coppice Willow and Its Independence from Biomass Yield. Bioenerg. Res. 3, 251–261 (2010). https://doi.org/10.1007/s12155-010-9077-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-010-9077-3