Abstract
Objective
The production of neurotoxic β-amyloid and the formation of hyperphosphorylated tau are thought to be critical steps contributing to the neuropathological mechanisms in Alzheimer’s disease (AD). However, there remains an argument as to their importance in the onset of AD. Recent studies have shown that axonopathy is considered as an early stage of AD. However, the exact relationship between axonopathy and the origin and development of classic neuropathological changes such as senile plaques (SPs) and neurofibrillary tangles (NFTs) is unclear. The present study aimed to investigate this relationship.
Methods
Postmortem tracing, combined with the immunohistochemical or immunofluorescence staining, was used to detect axonopathy and the formation of SPs and NFTs.
Results
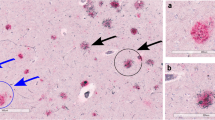
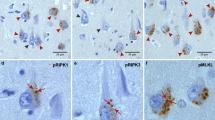
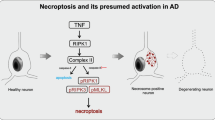
“Axonal leakage”-a novel type of axonopathy, was usually accompanied with the extensive swollen axons and varicosities, and was associated with the origin and development of Aβ plaques and hyperphosphorylated tau in the brains of AD patients.
Conclusion
Axonopathy, particularly axonal leakage, might be a key event in the initiation of the neuropathological processes in AD.
摘要
目的
神经毒性β-淀粉样蛋白沉积和tau蛋白过度磷酸化是阿尔茨海默病(Alzheimer’s disease, AD)神经病理机制的两个关键阶段, 然而关于它们在启动AD病程的重要性上仍存在争议。 最近研究显示, 轴突病变发生于AD 的早期, 然而轴突病变与老年斑(senile plaques, SPs)和神经纤维缠结(neurofibrillary tangles, NFTs)的发生发展之间的确切关系仍不清楚, 本研究旨在揭示它们之间的联系。
方法
运用神经示踪技术结合免疫组织化学、 免疫组织化学和免疫荧光双标技术, 研究AD患者大脑中的轴突病变以及SPs和NFTs的形成情况。
结果
AD患者的脑组织中呈现出一种新形式的轴突病变—“轴突漏”, 并伴随有大量肿胀的轴突和膨体。 此外, 轴突漏与-淀粉样蛋白沉积和tau蛋白过度磷酸化的形成及发展有关。
结论
轴突病变尤其是“轴突漏”可能是起始AD神经病理进程的一个关键事件。
Similar content being viewed by others
References
Masliah E, Mallory M, Deerinck T, DeTeresa R, Lamont S, Miller A, et al. Re-evaluation of the structural organization of neuritic plaques in Alzheimer’s disease. J Neuropathol Exp Neurol 1993, 52: 619–632.
Goedert M. Tau protein and the neurofibrillary pathology of Alzheimer’s disease. Trends Neurosci 1993, 16: 460–465.
Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci 1991, 12: 383–388.
Armstrong RA, Myers D, Smith CUM. The spatial patterns of plaques and tangles in Alzheimer’s disease do not support the ‘Cascade hypothesis’. Dementia 1993, 4: 16–20.
Terry RD. The pathogenesis of Alzheimer disease: an alternative to the amyloid hypothesis. J Neuropathol Exp Neurol 1996, 55: 1023–1025.
Neve RL, Robakis NK. Alzheimer’s disease: a re-examination of the amyloid hypothesis. Trends Neurosci 1998, 21: 15–19.
Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 2002, 297: 353–356.
Mudher A, Lovestone S. Alzheimer’s disease-do tauists and bap tists finally shake hands? Trends Neurosci 2002, 25: 22–26.
Duyckaerts C. Looking for the link between plaques and tangles. Neurobiol Aging 2004, 25: 735–739.
Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430: 631–639.
Schönheit B, Zarski R, Ohm TG. Spatial and temporal relationships between plaques and tangles in Alzheimer-pathology. Neurobiol Aging 2004, 25: 697–711.
Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell 2005, 120: 545–555.
Trojanowski JQ, Lee VMY. The Alzheimer’s brain binding out what’s broken tells us how to fix it. Am J Pathol 2005, 167: 1183–1188.
Armstrong RA. The pathogenesis of Alzheimer’s disease: a reevaluation of the “amyloid cascade hypothesis”. Int J Alzheimers Dis 2011, 2011: 630865.
Schwartz JH. Axonal transport: components, mechanisms, and specificity. Annu Rev Neurosci 1979, 2: 467–504.
Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 1998, 279: 519–526.
Dai J, Buijs RM, Kamphorst W, Swaab DF. Impaired axonal transport of cortical neurons in Alzheimer’s disease is associated with neuropathological changes. Brain Res 2002, 948: 138–144.
Dai J, Buijs RM, Swaab DF. Glucocorticoid hormone (cortisol) affects axonal transport in human cortex neurons but shows resistance in Alzheimer’s disease. Br J Pharmacol 2004, 143: 606–610.
Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science 2005, 307: 1282–1288.
Stokin GB, Goldstein LS. Axonal transport and Alzheimer’s disease. Annu Rev Biochem 2006, 75: 607–627.
Smith KD, Kallhoff V, Zheng H, Pautler RG. In vivo axonal transport rates decrease in a mouse model of Alzheimer’s disease. Neuroimage 2007, 35: 1401–1408.
Lazarov O, Morfini GA, Pigino G, Gadadhar A, Chen X, Robinson J, et al. Impairments in fast axonal transport and motor neuron deficits in transgenic mice expressing familial Alzheimer’s diseaselinked mutant presenilin 1. J Neurosci 2007, 27: 7011–7020.
Minoshima S, Cross D. In vivo imaging of axonal transport using MRI: aging and Alzheimer’s disease. Eur J Nucl Med Mol Imaging 2008, 35(Suppl 1): S89–92.
Adalbert R, Nogradi A, Babetto E, Janeckova L, Walker SA, Kerschensteiner M, et al. Severely dystrophic axons at amyloid plaques remain continuous and connected to viable cell bodies. Brain 2008, 132: 402–416.
Vickers JC, King AE, Woodhouse A, Kirkcaldie MT, Staal JA, McCormack GH, et al. Axonopathy and cytoskeletal disruption in degenerative diseases of the central nervous system. Brain Res Bull 2009, 80: 217–223.
Muresan V, Muresan Z. Is abnormal axonal transport a cause, a contributing factor or a consequence of the neuronal pathology in Alzheimer’s disease? Future Neurol 2009, 4: 761–773.
Massaad CA, Amin SK, Hu L, Mei Y, Klann E, Pautler RG. Mitochondrial superoxide contributes to blood flow and axonal transport deficits in the Tg2576 mouse model of Alzheimer’s disease. PLoS One 2010, 5: e10561.
Xiao A, Dai J. Axonal leakage is a key neuropathological change in Alzheimer’s disease. Acta Med Univ Sci Technol Huazhong 2006, 35: 277–278 (in Chinese).
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 1984, 34: 939–944.
Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol 1991, 1: 213–216.
Dai J, Swaab DF, Van der Vliet J, Buijs RM. Postmortem tracing reveals the organization of hypothalamic projections of the suprachiasmatic nucleus in the human brain. J Comp Neurol 1998, 400: 87–102.
Erisir A, Aoki C. A method of combining biocytin tract-tracing with avidin-biotin-peroxidase complex immunocytochemistry for pre-embedding electron microscopic labeling in neonatal tissue. J Neurosci Methods 1998, 81: 189–197.
Nunan J, Small DH. Regulation of APP cleavage by α-,β-, and γ -secretases. FEBS Lett 2000, 483: 6–10.
Shah SB, Nolan R, Davis E, Stokin GB, Niesman I, Canto I, et al. Examination of potential mechanisms of amyloid-induced defects in neuronal transport. Neurobiol Dis 2009, 36: 11–25.
Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, et al. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature 2008, 415: 720–725.
Steiner B, Mandelkow EM, Biernat J, Gustke N, Meyer HE, Schmidt B, et al. Phosphorylation of microtubule-associated protein tau: identification of the site for Ca2+-calmodulin dependent kinase and relationship with tau phosphorylation in Alzheimer tangles. EMBO J 1990, 9: 3539–3544.
Fleming LM, Johnson GV. Modulation of the phosphorylation state of tau in situ: the roles of calcium and cyclic AMP. Biochem J 1995, 309: 41–47.
Cras P, Kawai M, Lowery D, Gonzalez-DeWhitt P, Greenberg B, Perry G. Senile plaque neurites in Alzheimer disease accumulate amyloid precursor protein. Proc Natl Acad Sci U S A 1991, 88: 7552–7556.
Rifenburg RP, Perry G. Dystrophic neurites define diffuse as well as core-containing senile plaques in Alzheimer’s disease. Neurodegeneration 1995, 4: 235–237.
Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443: 787–795.
Muhammad S, Bierhaus A, Schwaninger M. Reactive oxygen species in diabetes-induced vascular damage, stroke, and Alzheimer’s disease. J Alzheimers Dis 2009, 16: 775–785.
Salmina AB. Neuron-glia interactions as therapeutic targets in neurodegeneration. J Alzheimers Dis 2009, 16: 485–502.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, AW., He, J., Wang, Q. et al. The origin and development of plaques and phosphorylated tau are associated with axonopathy in Alzheimer’s disease. Neurosci. Bull. 27, 287–299 (2011). https://doi.org/10.1007/s12264-011-1736-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-011-1736-7