Abstract
Background
Recently, the desmoplastic reaction has been implicated as having an important function in epithelial solid tumor biology. There have been no reports showing the relativity of invasive breast cancer and the desmoplastic reaction by a quantitative analysis of the myofibroblasts that were an important player in the desmoplastic reaction. The purpose of this study was to immunohistochemically investigate the correlation between the desmoplastic reaction and the clinicopathology of invasive breast cancer.
Methods
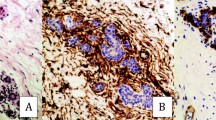
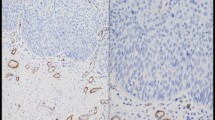
The study included 60 patients with a known prognosis of invasive breast cancer. We quantified the expression of α-SMA as a marker of myofibroblasts in the invasive breast cancer. After staining samples for α-SMA, their expression was extracted and quantified as a relative percentage by computer-assisted image analysis.
Results
There was relatively wide variation in the expression of α-SMA with the percentage of the area from 0.68 to 28.15% (mean 8.48 ± 5.40%). The metastasis group showed significantly higher α-SMA expression compared with the no metastasis group (p < 0.001). When the patients were divided into two groups according to their α-SMA expression using a cutoff point at the mean value of 8.48%, the high α-SMA group had a significantly poorer overall survival rate (p < 0.001). Multivariate analysis demonstrated that α-SMA and lymph node metastasis were identified as independent predictive factors of metastasis.
Conclusion
Myofibroblasts represent an important prognostic factor for invasive growth that is translated into a poor clinical prognosis for patients with invasive breast cancer.





Similar content being viewed by others
References
van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009.
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumors. Nature. 2000;406:747–52.
van‘t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6.
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74.
Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:206–14.
Chang HY, Nuyten DS, Sneddon JB, Hastie T, Tibshirani R, Sørlie T, et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci USA. 2005;102:3738–43.
Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9.
Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–9.
Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509–17.
De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429–47.
Barsky SH, Green WR, Grotendorst GR, Liotta LA. Desmoplastic breast carcinoma as a source of human myofibroblasts. Am J Pathol. 1984;115:329–33.
Yen TW, Aardal NP, Bronner MP, Thorning DR, Savard CE, Lee SP, et al. Myofibroblasts are responsible for the desmoplastic reaction surrounding human pancreatic carcinomas. Surgery. 2002;131:129–34.
Yamamoto H, Kondo M, Nakamori S, Nagano H, Wakasa K, Sugita Y, et al. JTE-522, a cyclooxygenase-2 inhibitor, is an effective chemopreventive agent against rat experimental liver fibrosis. Gastroenterology. 2003;125:556–71.
Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640–6.
De Wever O, Demetter P, Mareel M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–38.
Mahadevan D, Von Hoff DD. Tumor–stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186–97.
Matubara D, Morikawa T, Goto A, Nakajima J, Fukayama M, Niki T. Subepithelial myofibroblast in lung adenomarcinoma: a histological indicator of excellent prognosis. Mod Pathol. 2009;22:776–85.
Kawashiri S, Tanaka A, Noguchi N, Hase T, Nakaya H, Ohara T, et al. Significance of stromal desmoplasia and myofibroblast appearance at the invasive front in squamous cell carcinoma of the oral cavity. Otorhinolaryngology. 2009;31:1346–53.
Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–11.
Tsujino T, Seshimo I, Yamamoto H, Ngan CY, Ezumi K, Takemasa I, et al. Stromal myofibroblasts predict disease recurrence for corectal cancer. Clin Cancer Res. 2007;13:2082–90.
Hasebe T, Sasaki S, Imoto S, Ochiai A. Proliferative activity of intratumoral fibroblasts is closely correlated with lymph node and distant organ metastasis of invasive ductal carcinoma of the breast. Am J Pathol. 2000;156:1701–10.
Yazhou C, Wenlv S, Weidong Z, Licun W. Clinicopathological significance of stromal myofibroblasts in invasive ductal carcinoma of the breast. Tumor Biol. 2004;25:290–5.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Yamashita, M., Ogawa, T., Zhang, X. et al. Role of stromal myofibroblasts in invasive breast cancer: stromal expression of alpha-smooth muscle actin correlates with worse clinical outcome. Breast Cancer 19, 170–176 (2012). https://doi.org/10.1007/s12282-010-0234-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-010-0234-5