Abstract
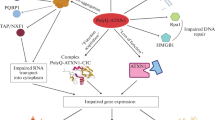
Spinocerebellar ataxia type 2 (SCA2) is an autosomal dominantly inherited, neurodegenerative disease. It can manifest either with a cerebellar syndrome or as Parkinson’s syndrome, while later stages involve mainly brainstem, spinal cord and thalamus. This particular atrophy pattern resembles sporadic multi-system-atrophy (MSA) and results in some clinical features indicative of SCA2, such as early saccade slowing, early hyporeflexia, severe tremor of postural or action type, and early myoclonus. For treatment, levodopa is temporarily useful for rigidity/bradykinesia and for tremor, magnesium for muscle cramps, but neuroprotective therapy will depend on the elucidation of pathogenesis. The disease cause lies in the polyglutamine domain of the protein ataxin-2, which can expand in families over successive generations resulting in earlier onset age and faster progression. Genetic testing in SCA2 and other polyglutamine disorders like the well-studied Huntington’s disease is now readily available for family planning. Although these disorders differ clinically and in the affected neuron populations, it is not understood how the different polyglutamine proteins mediate such tissue specificity. The neuronal intranuclear inclusion bodies described in other polyglutamine disorders are not frequent in SCA2. For the quite ubiquitously expressed ataxin-2, a subcellular localization at the Golgi, the endoplasmic reticulum and the plasma membrane, in interaction with proteins of mRNA translation and of endocytosis have been observed. As a first victim of SCA2 degeneration, cerebellar Purkinje neurons may be preferentially susceptible to alterations of these subcellular pathways, and therefore our review aims to portray the particular profile of the SCA2 disease process and correlate it to the specific features of ataxin-2.
Similar content being viewed by others
References
Wadia NH, Swami RK. A new form of heredo-familial spinocerebellar degeneration with slow eye movements (nine families). Brain. 1971;94:359–74.
Orozco G, Estrada R, Perry TL, Arana J, Fernandez R, Gonzalez-Quevedo A, et al. Dominantly inherited olivopontocerebellar atrophy from Eastern Cuba: Clinical, neuropathological, and biochemical findings. J Neurol Sci. 1989;93:37–50.
Auburger G, Diaz GO, Capote RF, Sanchez SG, Perez MP, del Cueto ME, et al. Autosomal dominant ataxia: Genetic evidence for locus heterogeneity from a Cuban foundereffect population. Am J Hum Genet. 1990;46:1163–77.
Orozco Diaz G, Nodarse Fleites A, Cordoves Sagaz R, Auburger G. Autosomal dominant cerebellar ataxia: Clinical analysis of 263 patients from a homogeneous population in Holguin, Cuba. Neurology. 1990;40:1369–75.
Velazquez Perez L, Almaguer Mederos L, Santos Falcon N, Hechavarria R, Sanchez Cruz G, Paneque HM. [Spinocerebellar ataxia type 2 in Cuba. A study of the electrophysiological phenotype and its correlation with clinical and molecular variables]. Rev Neurol. 2001;33:1129–36.
Sasaki H, Fukazawa T, Wakisaka A, Hamada K, Hamada T, Koyama T, et al. Central phenotype and related varieties of spinocerebellar ataxia 2 (SCA2): A clinical and genetic study with a pedigree in the Japanese. J Neurol Sci. 1996;144:176–81.
Gwinn-Hardy K, Chen JY, Liu HC, Liu TY, Boss M, Seltzer W, et al. Spinocerebellar ataxia type 2 with parkinsonism in ethnic Chinese. Neurology. 2000;55:800–5.
Furtado S, Payami H, Lockhart PJ, Hanson M, Nutt JG, Singleton AA, et al. Profile of families with parkinsonismpredominant spinocerebellar ataxia type 2 (SCA2). Mov Disord. 2004;19:622–9.
Infante J, Berciano J, Volpini V, Corral J, Polo JM, Pascual J, et al. Spinocerebellar ataxia type 2 with Levodopa-responsive parkinsonism culminating in motor neuron disease. Mov Disord. 2004;19:848–52.
Lu CS, Chang HC, Kuo PC, Liu YL, Wu WS, Weng YH, et al. The parkinsonian phenotype of spinocerebellar ataxia type 3 in a Taiwanese family. Parkinsonism Relat Disord. 2004;10:369–73.
Shan DE, Liu RS, Sun CM, Lee SJ, Liao KK, Soong BW. Presence of spinocerebellar ataxia type 2 gene mutation in a patient with apparently sporadic Parkinson’s disease: Clinical implications. Mov Disord. 2004;19:1357–60.
Simon-Sanchez J, Hanson M, Singleton A, Hernandez D, McInerney A, Nussbaum R, et al. Analysis of SCA-2 and SCA-3 repeats in Parkinsonism: evidence of SCA-2 expansion in a family with autosomal dominant Parkinson’s disease. Neurosci Lett. 2005;382:191–4.
Sanchez-Cruz G, Velazquez-Perez L, Gomez-Pena L, Martinez-Gongora E, Castellano-Sanchez G, Santos-Falcon N. [Dysautonomic features in patients with Cuban type 2 spinocerebellar ataxia]. Rev Neurol. 2001;33:428–34.
Trojano L, Chiacchio L, Grossi D, Pisacreta AI, Calabrese O, Castaldo I, et al. Determinants of cognitive disorders in Autosomal Dominant Cerebellar Ataxia type 1. J Neurol Sci. 1998;157:162–7.
Storey E, Forrest SM, Shaw JH, Mitchell P, Gardner RJ. Spinocerebellar ataxia type 2: clinical features of a pedigree displaying prominent frontal-executive dysfunction. Arch Neurol. 1999;56:43–50.
Le Pira F, Zappala G, Saponara R, Domina E, Restivo D, Reggio E, et al. Cognitive findings in spinocerebellar ataxia type 2: Relationship to genetic and clinical variables. J Neurol Sci. 2002;201:53–7.
Burk K, Globas C, Bosch S, Klockgether T, Zuhlke C, Daum I, et al. Cognitive deficits in spinocerebellar ataxia type 1, 2, and 3. J Neurol. 2003;250:207–11.
Reynaldo-Arminan RD, Reynaldo-Hernandez R, Paneque-Herrera M, Prieto-Avila L, Perez-Ruiz E. [Mental disorders in patients with spinocerebellar ataxia type 2 in Cuba]. Rev Neurol. 2002;35:818–21.
Klockgether T, Ludtke R, Kramer B, Abele M, Burk K, Schols L, et al. The natural history of degenerative ataxia: a retrospective study in 466 patients. Brain. 1998;121(Pt 4):589–600.
Maschke M, Oehlert G, Xie TD, Perlman S, Subramony SH, Kumar N, et al. Clinical feature profile of spinocerebellar ataxia type 1-8 predicts genetically defined subtypes. Mov Disord. 2005;20:1405–12.
Cancel G, Durr A, Didierjean O, Imbert G, Burk K, Lezin A, et al. Molecular and clinical correlations in spinocerebellar ataxia 2: A study of 32 families. Hum Mol Genet. 1997;6:709–15.
Schols L, Gispert S, Vorgerd M, Menezes Vieira-Saecker AM, Blanke P, Auburger G, et al. Spinocerebellar ataxia type 2. Genotype and phenotype in German kindreds. Arch Neurol. 1997;54:1073–80.
Sasaki H, Wakisaka A, Sanpei K, Takano H, Igarashi S, Ikeuchi T, et al. Phenotype variation correlates with CAG repeat length in SCA2 – a study of 28 Japanese patients. J Neurol Sci. 1998;159:202–8.
Filla A, De Michele G, Santoro L, Calabrese O, Castaldo I, Giuffrida S, et al. Spinocerebellar ataxia type 2 in southern Italy: a clinical and molecular study of 30 families. J Neurol. 1999;246:467–71.
Velazquez-Perez L, Seifried C, Santos-Falcon N, Abele M, Ziemann U, Almaguer LE, et al. Saccade velocity is controlled by polyglutamine size in spinocerebellar ataxia 2. Ann Neurol. 2004;56:444–7.
De Rosa A, Striano P, Barbieri F, De Falco A, Rinaldi C, Tucci T, et al. Suppression of myoclonus in SCA2 by piracetam. Mov Disord. 2005;21:116–8.
Babovic-Vuksanovic D, Snow K, Patterson MC, Michels VV. Spinocerebellar ataxia type 2 (SCA 2) in an infant with extreme CAG repeat expansion. Am J Med Genet. 1998;79:383–7.
Rufa A, Dotti MT, Galli L, Orrico A, Sicurelli F, Federico A. Spinocerebellar ataxia type 2 (SCA2) associated with retinal pigmentary degeneration. Eur Neurol. 2002;47:128–9.
Tan NC, Zhou Y, Tan AS, Chong SS, Lee WL. Spinocerebellar ataxia type 2 with focal epilepsy–an unusual association. Ann Acad Med Singapore. 2004;33:103–6.
Mao R, Aylsworth AS, Potter N, Wilson WG, Breningstall G, Wick MJ, et al. Childhood-onset ataxia: testing for large CAG-repeats in SCA2 and SCA7. Am J Med Genet. 2002;110:338–45.
Rivaud-Pechoux S, Durr A, Gaymard B, Cancel G, Ploner CJ, Agid Y, et al. Eye movement abnormalities correlate with genotype in autosomal dominant cerebellar ataxia type I. Ann Neurol. 1998;43:297–302.
Burk K, Fetter M, Abele M, Laccone F, Brice A, Dichgans J, et al. Autosomal dominant cerebellar ataxia type I: Oculomotor abnormalities in families with SCA1, SCA2, and SCA3. J Neurol. 1999;246:789–97.
Kubis N, Durr A, Gugenheim M, Chneiweiss H, Mazzetti P, Brice A, et al. Polyneuropathy in autosomal dominant cerebellar ataxias: Phenotype-genotype correlation. Muscle Nerve. 1999;22:712–7.
Velazquez-Perez L, Santos FN, Garcia R, Paneque HM, Hechavarria PR. [Epidemiology of Cuban hereditary ataxia]. Rev Neurol. 2001;32:606–11.
van de Warrenburg BP, Notermans NC, Schelhaas HJ, van Alfen N, Sinke RJ, Knoers NV, et al. Peripheral nerve involvement in spinocerebellar ataxias. Arch Neurol. 2004;61:257–61.
Perretti A, Santoro L, Lanzillo B, Filla A, De Michele G, Barbieri F, et al. Autosomal dominant cerebellar ataxia type I: multimodal electrophysiological study and comparison between SCA1 and SCA2 patients. J Neurol Sci. 1996;142:45–53.
Abele M, Burk K, Andres F, Topka H, Laccone F, Bosch S, et al. Autosomal dominant cerebellar ataxia type I. Nerve conduction and evoked potential studies in families with SCA1, SCA2 and SCA3. Brain. 1997;120(Pt 12):2141–8.
Yokota T, Sasaki H, Iwabuchi K, Shiojiri T, Yoshino A, Otagiri A, et al. Electrophysiological features of central motor conduction in spinocerebellar atrophy type 1, type 2, and Machado-Joseph disease. J Neurol Neurosurg Psychiatry. 1998;65:530–4.
Schwenkreis P, Tegenthoff M, Witscher K, Bornke C, Przuntek H, Malin JP, et al. Motor cortex activation by transcranial magnetic stimulation in ataxia patients depends on the genetic defect. Brain. 2002;125:301–9.
Restivo DA, Lanza S, Giuffrida S, Le Pira F, Drago MT, Di Mauro R, et al. Cortical silent period prolongation in spinocerebellar ataxia type 2 (SCA2). Funct Neurol. 2004;19:37–41.
Boesch SM, Frauscher B, Brandauer E, Wenning GK, Hogl B, Poewe W. Disturbance of rapid eye movement sleep in spinocerebellar ataxia type 2. Mov Disord. 2006;21:1751–4.
Tuin I, Voss U, Kang J-S, Kessler K, Rüb U, Nolte D, et al. Stages of sleep pathology in spinocerebellar ataxia type 2 (SCA2). Neurology. 2006;67:1966–72.
Rakowicz M, Zdzienicka E, Poniatowska R, Waliniowska E, Sulek A, Jakubowska T, et al. [Spinocerebellar ataxias type 1 and 2: comparison of clinical, electrophysiological and magnetic resonance evaluation.]. Neurol Neurochir Pol. 2005;39:1263–275.
Burk K, Abele M, Fetter M, Dichgans J, Skalej M, Laccone F, et al. Autosomal dominant cerebellar ataxia type I clinical features and MRI in families with SCA1, SCA2 and SCA3. Brain. 1996;119(Pt 5):1497–505.
Giuffrida S, Saponara R, Restivo DA, Trovato Salinaro A, Tomarchio L, Pugliares P, et al. Supratentorial atrophy in spinocerebellar ataxia type 2: MRI study of 20 patients. J Neurol. 1999;246:383–8.
Brenneis C, Bosch SM, Schocke M, Wenning GK, Poewe W. Atrophy pattern in SCA2 determined by voxelbased morphometry. Neuroreport. 2003;14:1799–802.
Inagaki A, Iida A, Matsubara M, Inagaki H. Positron emission tomography and magnetic resonance imaging in spinocerebellar ataxia type 2: A study of symptomatic and asymptomatic individuals. Eur J Neurol. 2005;12:725–8.
Brenneis C, Boesch SM, Egger KE, Seppi K, Scherfler C, Schocke M, et al. Cortical atrophy in the cerebellar variant of multiple system atrophy: A voxel-based morphometry study. Mov Disord. 2005;21:159–65.
Boesch SM, Donnemiller E, Muller J, Seppi K, Weirich-Schwaiger H, Poewe W, et al. Abnormalities of dopaminergic neurotransmission in SCA2: a combined 123I-betaCIT and 123I-IBZM SPECT study. Mov Disord. 2004;19:1320–5.
Wullner U, Reimold M, Abele M, Burk K, Minnerop M, Dohmen BM, et al. Dopamine transporter positron emission tomography in spinocerebellar ataxias type 1, 2, 3, and 6. Arch Neurol. 2005;62:1280–5.
Boesch SM, Schocke M, Burk K, Hollosi P, Fornai F, Aichner FT, et al. Proton magnetic resonance spectroscopic imaging reveals differences in spinocerebellar ataxia types 2 and 6. J Magn Reson Imaging. 2001;13:553–9.
Estrada R, Galarraga J, Orozco G, Nodarse A, Auburger G. Spinocerebellar ataxia 2 (SCA2): morphometric analyses in 11 autopsies. Acta Neuropathol (Berl). 1999;97:306–10.
Gierga K, Burk K, Bauer M, Orozco Diaz G, Auburger G, Schultz C, et al. Involvement of the cranial nerves and their nuclei in spinocerebellar ataxia type 2 (SCA2). Acta Neuropathol (Berl). 2005;109:617–31.
Rub U, Del Turco D, Burk K, Diaz GO, Auburger G, Mittelbronn M, et al. Extended pathoanatomical studies point to a consistent affection of the thalamus in spinocerebellar ataxia type 2. Neuropathol Appl Neurobiol. 2005;31:127–40.
Rub U, Seidel K, Ozerden I, Gierga K, Brunt ER, Schols L, et al. Consistent affection of the central somatosensory system in spinocerebellar ataxia type 2 and type 3 and its significance for clinical symptoms and rehabilitative therapy. Brain Res Rev. 2007;53:235–49.
Ying SH, Choi SI, Lee M, Perlman SL, Baloh RW, Toga AW, et al. Relative atrophy of the flocculus and ocular motor dysfunction in SCA2 and SCA6. Ann N Y Acad Sci. 2005;1039:430–5.
Rub U, Gierga K, Brunt ER, de Vos RA, Bauer M, Schols L, et al. Spinocerebellar ataxias types 2 and 3: degeneration of the precerebellar nuclei isolates the three phylogenetically defined regions of the cerebellum. J Neural Transm. 2005;112:1523–45.
Rub U, Brunt ER, Petrasch-Parwez E, Schols L, Theegarten D, Auburger G, et al. Degeneration of ingestion-related brainstem nuclei in spinocerebellar ataxia type 2, 3, 6 and 7. Neuropathol Appl Neurobiol. 2006;32:635–49.
Huynh DP, Del Bigio MR, Ho DH, Pulst SM. Expression of ataxin-2 in brains from normal individuals and patients with Alzheimer’s disease and spinocerebellar ataxia 2. Ann Neurol. 1999;45:232–41.
Rub U, Schultz C, Del Tredici K, Gierga K, Reifenberger G, de Vos RA, et al. Anatomically based guidelines for systematic investigation of the central somatosensory system and their application to a spinocerebellar ataxia type 2 (SCA2) patient. Neuropathol Appl Neurobiol. 2003;29:418–33.
Malandrini A, Galli L, Villanova M, Palmeri S, Parrotta E, DeFalco D, et al. CAG repeat expansion in an italian family with spinocerebellar ataxia type 2 (SCA2): A clinical and genetic study. Eur Neurol. 1998;40:164–8.
Yagishita S, Inoue M. Clinicopathology of spinocerebellar degeneration: Its correlation to the unstable CAG repeat of the affected gene. Pathol Int. 1997;47:1–15.
Armstrong J, Bonaventura I, Rojo A, Gonzalez G, Corral J, Nadal N, et al. Spinocerebellar ataxia type 2 (SCA2) with white matter involvement. Neurosci Lett. 2005;381:247–51.
Berciano J, Ferrer I. Glial cell cytoplasmic inclusions in SCA2 do not express alpha-synuclein. J Neurol. 2005;252:742–4.
Koyano S, Iwabuchi K, Yagishita S, Kuroiwa Y, Uchihara T. Paradoxical absence of nuclear inclusion in cerebellar Purkinje cells of hereditary ataxias linked to CAG expansion. J Neurol Neurosurg Psychiatry. 2002;73:450–2.
Pang JT, Giunti P, Chamberlain S, An SF, Vitaliani R, Scaravilli T, et al. Neuronal intranuclear inclusions in SCA2: a genetic, morphological and immunohistochemical study of two cases. Brain. 2002;125:656–63.
Uchihara T, Fujigasaki H, Koyano S, Nakamura A, Yagishita S, Iwabuchi K. Non-expanded polyglutamine proteins in intranuclear inclusions of hereditary ataxias – triple-labeling immunofluorescence study. Acta Neuropathol (Berl). 2001;102:149–52.
Huynh DP, Figueroa K, Hoang N, Pulst SM. Nuclear localization or inclusion body formation of ataxin-2 are not necessary for SCA2 pathogenesis in mouse or human. Nat Genet. 2000;26:44–50.
Gispert S, Twells R, Orozco G, Brice A, Weber J, Heredero L, et al. Chromosomal assignment of the second locus for autosomal dominant cerebellar ataxia (SCA2) to chromosome 12q23–24.1. Nat Genet. 1993;4:295–9.
Pulst SM, Nechiporuk A, Nechiporuk T, Gispert S, Chen XN, Lopes-Cendes I, et al. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet. 1996;14:269–76.
Imbert G, Saudou F, Yvert G, Devys D, Trottier Y, Garnier JM, et al. Cloning of the gene for spinocerebellar ataxia 2 reveals a locus with high sensitivity to expanded CAG/glutamine repeats. Nat Genet. 1996;14:285–91.
Sanpei K, Takano H, Igarashi S, Sato T, Oyake M, Sasaki H, et al. Identification of the spinocerebellar ataxia type 2 gene using a direct identification of repeat expansion and cloning technique, DIRECT. Nat Genet. 1996;14:277–84.
Pulst SM, Santos N, Wang D, Yang H, Huynh D, Velazquez L, et al. Spinocerebellar ataxia type 2: polyQ repeat variation in the CACNA1A calcium channel modifies age of onset. Brain. 2005;128:2297–303.
Pujana MA, Corral J, Gratacos M, Combarros O, Berciano J, Genis D, et al. Spinocerebellar ataxias in Spanish patients: Genetic analysis of familial and sporadic cases. The Ataxia Study Group. Hum Genet. 1999;104:516–22.
Sahba S, Nechiporuk A, Figueroa KP, Nechiporuk T, Pulst SM. Genomic structure of the human gene for spinocerebellar ataxia type 2 (SCA2) on chromosome 12q24.1. Genomics. 1998;47:359–64.
Affaitati A, de Cristofaro T, Feliciello A, Varrone S. Identification of alternative splicing of spinocerebellar ataxia type 2 gene. Gene. 2001;267:89–93.
Nechiporuk T, Huynh DP, Figueroa K, Sahba S, Nechiporuk A, Pulst SM. The mouse SCA2 gene: cDNA sequence, alternative splicing and protein expression. Hum Mol Genet. 1998;7:1301–9.
Albrecht M, Golatta M, Wullner U, Lengauer T. Structural and functional analysis of ataxin-2 and ataxin-3. Eur J Biochem. 2004;271:3155–70.
Neuwald AF, Koonin EV. Ataxin-2, global regulators of bacterial gene expression, and spliceosomal snRNP proteins share a conserved domain. J Mol Med. 1998;76:3–5.
Satterfield TF, Pallanck LJ. Ataxin-2 and its Drosophila homolog, ATX2, physically assemble with polyribosomes. Hum Mol Genet. 2006;15:2523–32.
Meunier C, Bordereaux D, Porteu F, Gisselbrecht S, Chretien S, Courtois G. Cloning and characterization of a family of proteins associated with Mpl. J Biol Chem. 2002;277:9139–47.
Shibata H, Huynh DP, Pulst SM. A novel protein with RNA-binding motifs interacts with ataxin-2. Hum Mol Genet. 2000;9:1303–13.
Huynh DP, Yang HT, Vakharia H, Nguyen D, Pulst SM. Expansion of the polyQ repeat in ataxin-2 alters its Golgi localization, disrupts the Golgi complex and causes cell death. Hum Mol Genet. 2003;12:1485–96.
Turnbull VJ, Storey E, Tarlac V, Walsh R, Stefani D, Clark R, et al. Different ataxin-2 antibodies display different immunoreactive profiles. Brain Res. 2004;1027:103–16.
Chen HK, Fernandez-Funez P, Acevedo SF, Lam YC, Kaytor MD, Fernandez MH, et al. Interaction of Aktphosphorylated ataxin-1 with 14-3-3 mediates neurodegeneration in spinocerebellar ataxia type 1. Cell. 2003;113:457–68.
Berg D, Holzmann C, Riess O. 14–3–3 proteins in the nervous system. Nat Rev Neurosci. 2003;4:752–62.
Ralser M, Albrecht M, Nonhoff U, Lengauer T, Lehrach H, Krobitsch S. An integrative approach to gain insights into the cellular function of human ataxin-2. J Mol Biol. 2005;346:203–14.
Bravo J, Aguilar-Henonin L, Olmedo G, Guzman P. Four distinct classes of proteins as interaction partners of the PABC domain of Arabidopsis thaliana Poly(A)-binding proteins. Mol Genet Genomics. 2005;272:651–65.
Kedersha N, Anderson P. Stress granules: Sites of mRNA triage that regulate mRNA stability and translatability. Biochem Soc Trans. 2002;30:963–9.
Villace P, Marion RM, Ortin J. The composition of Staufencontaining RNA granules from human cells indicates their role in the regulated transport and translation of messenger RNAs. Nucleic Acids Res. 2004;32:2411–20.
Ciosk R, DePalma M, Priess JR. ATX-2, the C. elegans ortholog of ataxin 2, functions in translational regulation in the germline. Development. 2004;131:4831–41.
Vernet C, Artzt K. STAR, a gene family involved in signal transduction and activation of RNA. Trends Genet. 1997;13:479–84.
Lim J, Hao T, Shaw C, Patel AJ, Szabo G, Rual JF, et al. A protein-protein interaction network for human inherited ataxias and disorders of Purkinje cell degeneration. Cell. 2006;125:801–14.
Ralser M, Nonhoff U, Albrecht M, Lengauer T, Wanker EE, Lehrach H, et al. Ataxin-2 and huntingtin interact with endophilin-A complexes to function in plastin-associated pathways. Hum Mol Genet. 2005;14:2893–909.
Micheva KD, Ramjaun AR, Kay BK, McPherson PS. SH3 domain-dependent interactions of endophilin with amphiphysin. FEBS Lett. 1997;414:308–12.
de Heuvel E, Bell AW, Ramjaun AR, Wong K, Sossin WS, McPherson PS. Identification of the major synaptojaninbinding proteins in brain. J Biol Chem. 1997;272:8710–6.
Soubeyran P, Kowanetz K, Szymkiewicz I, Langdon WY, Dikic I. Cbl-CIN85-endophilin complex mediates ligandinduced downregulation of EGF receptors. Nature. 2002;416:183–7.
Satterfield TF, Jackson SM, Pallanck LJ. A Drosophila homolog of the polyglutamine disease gene SCA2 is a dosage-sensitive regulator of actin filament formation. Genetics. 2002;162:1687–702.
Huynh DP, Scoles DR, Nguyen D, Pulst SM. The autosomal recessive juvenile Parkinson disease gene product, parkin, interacts with and ubiquitinates synaptotagmin XI. Hum Mol Genet. 2003;12:2587–97.
Imai Y, Soda M, Inoue H, Hattori N, Mizuno Y, Takahashi R. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell. 2001;105:891–902.
Huynh DP, Nguyen DT, Pulst-Korenberg JB, Brice A, Pulst SM. Parkin is an E3 ubiquitin-ligase for normal and mutant ataxin-2 and prevents ataxin-2-induced cell death. Exp Neurol. 2007;203:531–41.
Aguiar J, Fernandez J, Aguilar A, Mendoza Y, Vazquez M, Suarez J, et al. Ubiquitous expression of human SCA2 gene under the regulation of the SCA2 self promoter cause specific Purkinje cell degeneration in transgenic mice. Neurosci Lett. 2006;392:202–6.
Freund H-J, Barnikol UB, Nolte D, Treuer H, Auburger G, Tass PA, et al. Subthalamic-thalamic DBS in a case with spinocerebellar ataxia type 2 and severe tremor – an unusual clinical benefit. 2007;22:732–5.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lastres-Becker, I., Rüb, U. & Auburger, G. Spinocerebellar ataxia 2 (SCA2). Cerebellum 7, 115–124 (2008). https://doi.org/10.1007/s12311-008-0019-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-008-0019-y