Abstract
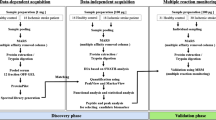
Tissue plasminogen activator (tPA) is the only FDA-approved medical therapy for acute ischemic stroke. But as a serine peptidase, intravenous tPA can affect the expression of other proteases that may be implicated in blood–brain barrier breakdown. Such parallel cascades of cell signaling may be involved in intracranial hemorrhage, the major side effect of tPA. Here, we describe an initial attempt in proteomic substrate profiling, i.e., degradomics in human plasma within the context of acute stroke. Plasma from acute stroke patients were analyzed pre- and post-intravenous tPA using tandem mass spectrometry and protein array profiling to identify substrates and proteases of interest. In non-tPA-treated stroke plasma, degradomic patterns indicated a rapid induction of protease activity within 3 h of stroke onset that mostly stabilized by 24 h. But in tPA-treated patients, pre- and post-tPA samples from the same patient demonstrated distinct degradomic patterns that persisted even up to 3–5 days after stroke onset. Matching control patients without strokes had little change in degradomic profiles over time. Our findings demonstrate that tPA treatment changes the plasma degradomic profiles in acute stroke patients. These composite proteolytic profiles may provide a glimpse of the pleiotropic effects of tPA on cellular signaling cascades at the bedside. This study supports the feasibility of performing pharmacoproteomics at the bedside, which may ultimately allow us to dissect mechanisms of thrombolysis-related therapeutic efficacy in stroke.


Similar content being viewed by others
References
The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–7
Tanne D, Bates VE, Verro P, Kasner SE, Binder JR, Patel SC, et al. Initial clinical experience with IV tissue plasminogen activator for acute ischemic stroke: a multicenter survey. The t-PA stroke survey group. Neurology. 1999;53:424–7.
Katzan IL, Furlan AJ, Lloyd LE, Frank JI, Harper DL, Hinchey JA, et al. Use of tissue-type plasminogen activator for acute ischemic stroke: the Cleveland area experience. JAMA. 2000;283:1151–8.
Marler JR, Goldstein LB. Stroke - tPA and the clinic. Science 2003;301:1677
Hill MD, Buchan AM. Methodology for the Canadian activase for stroke effectiveness study (cases). Cases investigators. Can J Neurol Sci. 2001;28:232–8.
Albers GW, Clark WM, Madden KP, Hamilton SA. Atlantis trial: results for patients treated within 3 hours of stroke onset. Alteplase thrombolysis for acute noninterventional therapy in ischemic stroke. Stroke. 2002;33:493–5.
Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–74.
Donnan GA, Hommel M, Davis SM, McNeil JJ. Streptokinase in acute ischaemic stroke. Steering Committees of the ASK and MAST-E trials. Australian Streptokinase Trial. Lancet. 1995;346:56.
Hommel M, Boissel JP, Cornu C, Boutitie F, Lees KR, Besson G, et al. Termination of trial of streptokinase in severe acute ischaemic stroke. MAST study group. Lancet. 1995;345:57.
Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS study: a randomized controlled trial. Alteplase thrombolysis for acute noninterventional therapy in ischemic stroke. JAMA. 1999;282:2019–26.
Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309.
Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–29.
Bluhmki E, Chamorro A, Davalos A, Machnig T, Sauce C, Wahlgren N, et al. Stroke treatment with alteplase given 3.0–4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol. 2009;8:1095–102.
Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–703.
Molina CA, Alvarez-Sabin J, Montaner J, Abilleira S, Arenillas JF, Coscojuela P, et al. Thrombolysis-related hemorrhagic infarction: a marker of early reperfusion, reduced infarct size, and improved outcome in patients with proximal middle cerebral artery occlusion. Stroke. 2002;33:1551–6.
Castellanos M, Leira R, Serena J, Pumar JM, Lizasoain I, Castillo J, et al. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34:40–6.
Montaner J, Molina CA, Monasterio J, Abilleira S, Arenillas JF, Ribo M, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107:598–603.
Ebinger M, Iwanaga T, Prosser JF, De Silva DA, Christensen S, Collins M, et al. Clinical-diffusion mismatch and benefit from thrombolysis 3 to 6 hours after acute stroke. Stroke. 2009;40:2572–4.
Butcher K, Christensen S, Parsons M, De Silva DA, Ebinger M, Levi C, et al. Postthrombolysis blood pressure elevation is associated with hemorrhagic transformation. Stroke. 2010;41:72–7.
De Silva DA, Brekenfeld C, Ebinger M, Christensen S, Barber PA, Butcher KS, et al. The benefits of intravenous thrombolysis relate to the site of baseline arterial occlusion in the Echoplanar Imaging Thrombolytic Evaluation Trial (epithet). Stroke. 2010;41:295–9.
De Silva DA, Ebinger M, Christensen S, Parsons MW, Levi C, Butcher K, et al. Baseline diabetic status and admission blood glucose were poor prognostic factors in the epithet trial. Cerebrovasc Dis. 2010;29:14–21.
Hemmen TM, Rapp KS, Emond JA, Raman R, Lyden PD. Analysis of the National Institute of Neurological Disorders and Stroke tissue plasminogen activator studies following European Cooperative Acute Stroke Study III patient selection criteria. J Stroke Cerebrovasc Dis. 2010;19:290–3.
Wang X, Lee SR, Arai K, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003;9:1313–7.
Montaner J, Alvarez-Sabin J, Molina CA, Angles A, Abilleira S, Arenillas J, et al. Matrix metalloproteinase expression is related to hemorrhagic transformation after cardioembolic stroke. Stroke. 2001;32:2762–7.
Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood–brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–32.
Rosenberg GA, Navratil M, Barone F, Feuerstein G. Proteolytic cascade enzymes increase in focal cerebral ischemia in rat. J Cereb Blood Flow Metab. 1996;16:360–6.
Pfefferkorn T, Rosenberg GA. Closure of the blood–brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34:2025–30.
Fujimura M, Gasche Y, Morita-Fujimura Y, Massengale J, Kawase M, Chan PH. Early appearance of activated matrix metalloproteinase-9 and blood–brain barrier disruption in mice after focal cerebral ischemia and reperfusion. Brain Res. 1999;842:92–100.
Hamann GF, Okada Y, del Zoppo GJ. Hemorrhagic transformation and microvascular integrity during focal cerebral ischemia/reperfusion. J Cereb Blood Flow Metab. 1996;16:1373–8.
Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke. 2000;31:3034–40.
Hosomi N, Lucero J, Heo JH, Koziol JA, Copeland BR, del Zoppo GJ. Rapid differential endogenous plasminogen activator expression after acute middle cerebral artery occlusion. Stroke. 2001;32:1341–8.
Abilleira S, Montaner J, Molina CA, Monasterio J, Castillo J, Alvarez-Sabin J. Matrix metalloproteinase-9 concentration after spontaneous intracerebral hemorrhage. J Neurosurg. 2003;99:65–70.
Ning M, Lo EH, Furie K, Koroshetz W, Sleeper L, Wang X, et al. T-pa-mediated increase in matrix metalloproteinase-9 in acute ischemic stroke is independent of inflammatory response. Stroke. 2004;35:298.
Heiss WD. Experimental evidence of ischemic thresholds and functional recovery. Stroke. 1992;23:1668–72.
Lopez-Otin C, Overall CM. Protease degradomics: a new challenge for proteomics. Nat Rev Mol Cell Biol. 2002;3:509–19.
Liotta LA, Petricoin EF. Serum peptidome for cancer detection: spinning biologic trash into diagnostic gold. J Clin Invest. 2006;116:26–30.
Liotta LA, Ferrari M, Petricoin E. Clinical proteomics: written in blood. Nature. 2003;425:905.
Van Eyk JE, Dunn MJ. Clinical proteomics: from diagnosis to therapy. New York: Wiley; 2008
Mun-Bryce S, Rosenberg GA. Matrix metalloproteinases in cerebrovascular disease. J Cereb Blood Flow Metab. 1998;18:1163–72.
Barr TL, Latour LL, Lee KY, Schaewe TJ, Luby M, Chang GS, et al. Blood–brain barrier disruption in humans is independently associated with increased matrix metalloproteinase-9. Stroke. 2004;41:e123–8.
Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood–brain barrier disruption. Stroke. 2004;35:2659–61.
Hornig CR, Busse O, Dorndorf W, Kaps M. Changes in csf blood–brain barrier parameters in ischaemic cerebral infarction. J Neurol. 1983;229:11–6.
Marchi N, Fazio V, Cucullo L, Kight K, Masaryk T, Barnett G, et al. Serum transthyretin monomer as a possible marker of blood-to-csf barrier disruption. J Neurosci. 2003;23:1949–55.
Pineda JA, Wang KK, Hayes RL. Biomarkers of proteolytic damage following traumatic brain injury. Brain Pathol. 2004;14:202–9.
Ning M, Wang X, Lo EH. Reperfusion injury after stroke: neurovascular proteases and the blood–brain barrier. Handb Clin Neurol. 2008;92:117–36.
Arrell DK, Neverova I, Van Eyk JE. Cardiovascular proteomics: evolution and potential. Circ Res. 2001;88:763–73.
Acknowledgments
This study is supported in part by NIH grants K23-NS51588, R21-NS52498, R01-NS67139, R37-NS37074, and P01-NS55104.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ning, M., Sarracino, D.A., Buonanno, F.S. et al. Proteomic Protease Substrate Profiling of tPA Treatment in Acute Ischemic Stroke Patients: A Step Toward Individualizing Thrombolytic Therapy at the Bedside. Transl. Stroke Res. 1, 268–275 (2010). https://doi.org/10.1007/s12975-010-0047-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-010-0047-z