Abstract
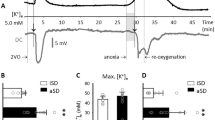
Constriction and dilation of large arteries of the brain regulates cerebral vascular resistance and cerebral microvascular pressure, which play key roles in regulation of cerebral circulation. We investigated the effect of ischemic stroke on vascular reactivity of the middle cerebral artery (MCA) using a rat transient focal cerebral ischemia model. Focal cerebral ischemia was induced by 1 h of MCA occlusion followed by reperfusion. MCAs were dissected from ischemic or contralateral hemisphere at 2 days or 2 weeks postreperfusion and mounted on two glass micropipettes for assessment of vascular reactivity. MCAs from the brains of sham surgeries were used as control. At 2 days postreperfusion, a significant alteration of myogenic reactivity was found in MCAs dissected from both ischemic and nonischemic hemispheres, which could still be identified at 2 weeks after reperfusion. Phenylephrine (PE) induced a remarkable vasoconstriction in MCAs from animals that underwent sham surgery. No significant alteration of vasoconstrictive response to PE was found in MCAs isolated from either ischemic or contralateral hemisphere at 2 days or 2 weeks after ischemic stroke, as compared with MCAs from sham animals. Acetylcholine (ACh) induced mild dilation in normal MCAs, which was reversed in MCAs from both ischemic and nonischemic hemispheres at 2 weeks after ischemic stroke. Sodium nitroprusside (SNP) induced vasodilation in MCAs from animals with sham operation, which was diminished in MCAs from both ischemic and nonischemic hemispheres at 2 days and 2 weeks after ischemic stroke. These results demonstrated that focal cerebral ischemia could induce long-term global cerebral vasculature dysfunction.





Similar content being viewed by others
References
Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415.
Savitz SI, Fisher M. Future of neuroprotection for acute stroke: in the aftermath of the SAINT trials. Ann Neurol. 2007;61:396–402.
Fisher M, Schaebitz W. An overview of acute stroke therapy: past, present, and future. Arch Intern Med. 2000;160:3196–206.
Faraci FM. Vascular protection. Stroke. 2003;34:327–9.
Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. 2004;35:2220–5.
Cipolla MJ, McCall AL, Lessov N, Porter JM. Reperfusion decreases myogenic reactivity and alters middle cerebral artery function after focal cerebral ischemia in rats. Stroke. 1997;28:176–80.
Cipolla MJ, Curry AB. Middle cerebral artery function after stroke: the threshold duration of reperfusion for myogenic activity. Stroke. 2002;33:2094–9.
Jimenez-Altayo F, Martin A, Rojas S, Justicia C, Briones AM, Giraldo J, Planas AM, Vila E. Transient middle cerebral artery occlusion causes different structural, mechanical, and myogenic alterations in normotensive and hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;293:H628–635.
Liu R, Wen Y, Perez E, Wang X, Day AL, Simpkins JW, Yang SH. 17beta-Estradiol attenuates blood–brain barrier disruption induced by cerebral ischemia-reperfusion injury in female rats. Brain Res. 2005;1060:55–61.
Taylor JC, Li Z, Yang HT, Laughlin MH, Terjung RL. Alpha-adrenergic inhibition increases collateral circuit conductance in rats following acute occlusion of the femoral artery. J Physiol. 2008;586:1649–67.
Taylor JC, Yang HT, Laughlin MH, Terjung RL. Alpha-adrenergic and neuropeptide Y Y1 receptor control of collateral circuit conductance: influence of exercise training. J Physiol. 2008;586:5983–98.
Shen Q, Du F, Huang S, Duong TQ. Spatiotemporal characteristics of postischemic hyperperfusion with respect to changes in T1, T2, diffusion, angiography, and blood–brain barrier permeability. J Cereb Blood Flow Metab. 2011;31:2076–85.
Cipolla MJ, Lessov N, Clark WM, Haley Jr EC. Postischemic attenuation of cerebral artery reactivity is increased in the presence of tissue plasminogen activator. Stroke. 2000;31:940–5.
Cipolla MJ, Lessov N, Hammer ES, Curry AB. Threshold duration of ischemia for myogenic tone in middle cerebral arteries: effect on vascular smooth muscle actin. Stroke. 2001;32:1658–64.
Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78:53–97.
Maneen MJ, Hannah R, Vitullo L, DeLance N, Cipolla MJ. Peroxynitrite diminishes myogenic activity and is associated with decreased vascular smooth muscle F-actin in rat posterior cerebral arteries. Stroke. 2006;37:894–9.
Butterworth RJ, Cluckie A, Jackson SH, Buxton-Thomas M, Bath PM. Pathophysiological assessment of nitric oxide (given as sodium nitroprusside) in acute ischaemic stroke. Cerebrovasc Dis. 1998;8:158–65.
Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–35.
Liu R YH, Yuan F, Yang SH. Neuroprotection targeting ischemic penumbra and beyond for the treatment of ischemic stroke. Neurological Research. 2012; in press.
Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–84.
Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, Kim D, Jahan R, Duckwiler GR, Yoon SR, Vinuela F, Liebeskind DS. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2008;79:625–9.
van Laar PJ, Hendrikse J, Klijn CJ, Kappelle LJ, van Osch MJ, van der Grond J. Symptomatic carotid artery occlusion: flow territories of major brain-feeding arteries. Radiology. 2007;242:526–34.
Villapol S, Bonnin P, Fau S, Baud O, Renolleau S, Charriaut-Marlangue C. Unilateral blood flow decrease induces bilateral and symmetric responses in the immature brain. Am J Pathol. 2009;175:2111–20.
Martinez-Revelles S, Jimenez-Altayo F, Caracuel L, Perez-Asensio FJ, Planas AM, Vila E. Endothelial dysfunction in rat mesenteric resistance artery after transient middle cerebral artery occlusion. J Pharmacol Exp Ther. 2008;325:363–9.
Kuroiwa T, Nagaoka T, Ueki M, Yamada I, Miyasaka N, Akimoto H. Different apparent diffusion coefficient: water content correlations of gray and white matter during early ischemia. Stroke. 1998;29:859–65.
Tatemichi TK, Desmond DW, Mayeux R, Paik M, Stern Y, Sano M, Remien RH, Williams JB, Mohr JP, Hauser WA, et al. Dementia after stroke: baseline frequency, risks, and clinical features in a hospitalized cohort. Neurology. 1992;42:1185–93.
Henon H, Durieu I, Guerouaou D, Lebert F, Pasquier F, Leys D. Poststroke dementia: incidence and relationship to prestroke cognitive decline. Neurology. 2001;57:1216–22.
Desmond DW, Moroney JT, Sano M, Stern Y. Incidence of dementia after ischemic stroke: results of a longitudinal study. Stroke. 2002;33:2254–60.
Altieri M, Di Piero V, Pasquini M, Gasparini M, Vanacore N, Vicenzini E, Lenzi GL. Delayed poststroke dementia: a 4-year follow-up study. Neurology. 2004;62:2193–7.
Yang SH, Shetty RA, Liu R, Sumien N, Heinrich KR, Rutledge M, Thangthaeng N, Brun-Zinkernagel AM, Forster MJ. Endovascular middle cerebral artery occlusion in rats as a model for studying vascular dementia. Age (Dordr). 2006;28:297–307.
Moody M, Panerai RB, Eames PJ, Potter JF. Cerebral and systemic hemodynamic changes during cognitive and motor activation paradigms. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1581–1588.
Atkinson J. Cerebrovascular structure and dementia: new drug targets. Trends Pharmacol Sci. 2001;22:630–5.
Acknowledgments
This study was supported partly by the NIH grants R01NS054687 (SY), R01NS054651 (SY), and R01DK079968 (RM). JCT was a postdoctoral trainee supported by T32 AG020494.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Winters, A., Taylor, J.C., Ren, M. et al. Transient Focal Cerebral Ischemia Induces Long-term Cerebral Vasculature Dysfunction in a Rodent Experimental Stroke Model. Transl. Stroke Res. 3, 279–285 (2012). https://doi.org/10.1007/s12975-012-0148-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-012-0148-y