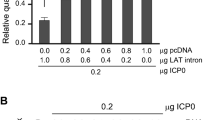
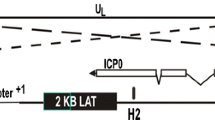
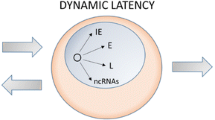
Abstract
Herpes simplex virus (HSV) establishes latent infections in sensory neurons from which it can periodically reactivate and cause recurrent disease and transmission to new hosts. Little is known about the virally encoded mechanisms that influence the maintenance of HSV latent infectious and modulate the frequency of virus reactivation from the latent state. Here, we report that the latency associated transcript locus of HSV-1 is required for long-term maintenance of reactivation competent latent infections.



Similar content being viewed by others
References
Arthur J, Efstathiou S et al (1993) Intranuclear foci containing low abundance herpes simplex virus latency-associated transcripts visualized by non-isotopic in situ hybridization. J Gen Virol 74(Pt 7):1363–1370
Bloom DC, Devi-Rao GB et al (1994) Molecular analysis of herpes simplex virus type 1 during epinephrine- induced reactivation of latently infected rabbits in vivo. J Virol 68(3):1283–1292
Deatly AM, Spivack JG et al (1987) RNA from an immediate early region of the type 1 herpes simplex virus genome is present in the trigeminal ganglia of latently infected mice. Proc Natl Acad Sci USA 84(10):3204–3208
Doerig C, Pizer LI et al (1991) An antigen encoded by the latency-associated transcript in neuronal cell cultures latently infected with herpes simplex virus type 1. J Virol 65(5):2724–2727
Farrell MJ, Dobson AT et al (1991) Herpes simplex virus latency-associated transcript is a stable intron. Proc Natl Acad Sci USA 88(3):790–794
Field HJ, Wildy P (1978) The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J Hyg (Lond) 81(2):267–277
Fuchs J, Celum C et al (2010) Clinical and virologic efficacy of herpes simplex virus type 2 suppression by acyclovir in a multicontinent clinical trial. J Infect Dis 201:1164–1168
Garber DA, Schaffer PA et al (1997) A LAT-associated function reduces productive-cycle gene expression during acute infection of murine sensory neurons with herpes simplex virus type 1. J Virol 71(8):5885–5893
Glynn JR, Biraro S et al (2009) Herpes simplex virus type 2: a key role in HIV incidence. AIDS 23(12):1595–1598
Green MT, Courtney RJ et al (1981) Detection of an immediate early herpes simplex virus type 1 polypeptide in trigeminal ganglia from latently infected animals. Infect Immun 34(3):987–992
Henderson G, Jaber T et al (2009) Identification of herpes simplex virus type 1 proteins encoded within the first 1.5 kb of the latency-associated transcript. J Neurovirol 15(5–6):439–448
Horsburgh BC, Chen SH et al (1998) Recurrent acyclovir-resistant herpes simplex in an immunocompromised patient: can strain differences compensate for loss of thymidine kinase in pathogenesis? J Infect Dis 178(3):618–625
Jaber T, Henderson G et al (2009) Identification of a novel herpes simplex virus type 1 transcript and protein (AL3) expressed during latency. J Gen Virol 90(Pt 10):2342–2352
Jacobson JG, Ruffner KL et al (1993) Herpes simplex virus thymidine kinase and specific stages of latency in murine trigeminal ganglia. J Virol 67(11):6903–6908
Kang W, Mukerjee R et al (2003) Establishment and maintenance of HSV latent infection is mediated through correct splicing of the LAT primary transcript. Virology 312(1):233–244
Peng W, Vitvitskaia O et al (2008) Identification of two small RNAs within the first 1.5-kb of the herpes simplex virus type 1-encoded latency-associated transcript. J Neurovirol 14(1):41–52
Pepose JS, Keadle TL et al (2006) Ocular herpes simplex: changing epidemiology, emerging disease patterns, and the potential of vaccine prevention and therapy. Am J Ophthalmol 141(3):547–557
Perng GC, Dunkel EC et al (1994) The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J Virol 68(12):8045–8055
Perng GC, Slanina SM et al (2000) The latency-associated transcript gene enhances establishment of herpes simplex virus type 1 latency in rabbits. J Virol 74(4):1885–1891
Perng GC, Maguen B et al (2002) A novel herpes simplex virus type 1 transcript (AL-RNA) antisense to the 5′ end of the latency-associated transcript produces a protein in infected rabbits. J Virol 76(16):8003–8010
Pyles RB, Thompson RL (1994) Mutations in accessory DNA replicating functions alter the relative mutation frequency of herpes simplex virus type 1 strains in cultured murine cells. J Virol 68(7):4514–4524
Rock DL, Nesburn AB et al (1987) Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol 61(12):3820–3826
Roizman B, Knipe DM, Whitley RJ (2007) Herpes Simplex Viruses. In: Knipe D, Howley P (eds) Fields Virology. Lippincott Williams & Wilkins, Philadelphia, pp 2501–2602
Sawtell NM (1997) Comprehensive quantification of herpes simplex virus latency at the single-cell level. J Virol 71(7):5423–5431
Sawtell NM (1998) The probability of in vivo reactivation of herpes simplex virus type 1 increases with the number of latently infected neurons in the ganglia. J Virol 72(8):6888–6892
Sawtell NM (2003) Quantitative analysis of herpes simplex virus reactivation in vivo demonstrates that reactivation in the nervous system is not inhibited at early times postinoculation. J Virol 77(7):4127–4138
Sawtell NM (2005) Detection and quantification of the rare latently infected cell undergoing herpes simplex virus transcriptional activation in the nervous system in vivo. Methods Mol Biol 292:57–72
Sawtell NM, Thompson RL (1992a) Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishment and reactivation from latency. J Virol 66(4):2157–2169
Sawtell NM, Thompson RL (1992b) Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J Virol 66(4):2150–2156
Sawtell NM, Thompson RL (2004) Comparison of herpes simplex virus reactivation in ganglia in vivo and in explants demonstrates quantitative and qualitative differences. J Virol 78(14):7784–7794
Sawtell NM, Poon DK et al (1998) The latent herpes simplex virus type 1 genome copy number in individual neurons is virus strain specific and correlates with reactivation. J Virol 72(7):5343–5350
Sawtell NM, Thompson RL et al (2006) Herpes simplex virus DNA synthesis is not a decisive regulatory event in the initiation of lytic viral protein expression in neurons in vivo during primary infection or reactivation from latency. J Virol 80(1):38–50
Sedarati F, Izumi KM et al (1989) Herpes simplex virus type 1 latency-associated transcription plays no role in establishment or maintenance of a latent infection in murine sensory neurons. J Virol 63(10):4455–4458
Stevens JG, Wagner EK et al (1987) RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science 235(4792):1056–1059
Stone MJ, Hawkins CP (2007) A medical overview of encephalitis. Neuropsychol Rehabil 17(4–5):429–449
Tenser RB, Miller RL et al (1979) Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus. Science 205(4409):915–917
Thomas SK, Gough G et al (1999) Herpes simplex virus latency-associated transcript encodes a protein which greatly enhances virus growth, can compensate for deficiencies in immediate-early gene expression, and is likely to function during reactivation from virus latency. J Virol 73(8):6618–6625
Thomas SK, Lilley CE et al (2002) A protein encoded by the herpes simplex virus (HSV) type 1 2-kilobase latency-associated transcript is phosphorylated, localized to the nucleus, and overcomes the repression of expression from exogenous promoters when inserted into the quiescent HSV genome. J Virol 76(8):4056–4067
Thompson RL, Sawtell NM (1997) The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J Virol 71(7):5432–5440
Thompson RL, Sawtell NM (2001) Herpes simplex virus type 1 latency-associated transcript gene promotes neuronal survival. J Virol 75(14):6660–6675
Thompson RL, Sawtell NM (2006) Evidence that the herpes simplex virus type 1 ICP0 protein does not initiate reactivation from latency in vivo. J Virol 80(22):10919–10930
Thompson RL, Shieh MT et al (2003) Analysis of herpes simplex virus ICP0 promoter function in sensory neurons during acute infection, establishment of latency, and reactivation in vivo. J Virol 77(22):12319–12330
Thompson RL, Preston CM et al (2009) De novo synthesis of VP16 coordinates the exit from HSV latency in vivo. PLoS Pathog 5(3):e1000352
Toma HS, Murina AT et al (2008) Ocular HSV-1 latency, reactivation and recurrent disease. Semin Ophthalmol 23(4):249–273
Umbach JL, Kramer MF et al (2008) MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature 454(7205):780–783
Wechsler SL, Nesburn AB et al (1988) Fine mapping of the latency-related gene of herpes simplex virus type 1: alternative splicing produces distinct latency-related RNAs containing open reading frames. J Virol 62(11):4051–4058
Acknowledgments
This project was supported by Award Number R01 EY 013168 from the National Eye Institute and RO1 AI 32121 of the National Institute of Allergy and Infectious Diseases. The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
All authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thompson, R.L., Sawtell, N.M. The herpes simplex virus type 1 latency associated transcript locus is required for the maintenance of reactivation competent latent infections. J. Neurovirol. 17, 552–558 (2011). https://doi.org/10.1007/s13365-011-0071-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-011-0071-0