Abstract
Background
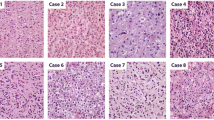
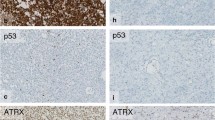
Glioblastomas are the most common and most malignant brain tumors in adults. A small subgroup of glioblastomas contains areas with histological features of oligodendroglial differentiation (GBMO). Our objective was to genetically characterize the oligodendroglial and the astrocytic parts of GBMOs and correlate morphologic and genetic features with clinical data.
Methods
The oligodendroglial and the “classic” glioblastoma parts of 13 GBMO were analyzed separately by interphase fluoreszence in situ hybridization (FISH) on paraffin sections using a custom probe set (regions 1p, 1q, 7q, 10q, 17p, 19q, cen18, 21q) and by comparative genomic hybridization (CGH) of microdissected paraffin embedded tumor tissue.
Results
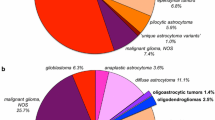
We identified four distinct genetic subtypes in 13 GBMOs: an “astrocytic” subtype (9/13) characterized by +7/-10; an “oligodendroglial” subtype with -1p/-19q (1/13); an “intermediate” subtype showing +7/-1p (1/13), and an “other” subtype having none of the former aberrations typical for gliomas (2/13). The different histological tumor parts of GBMO revealed common genetic changes in all tumors and showed additional aberrations specific for each part.
Conclusion
Our findings demonstrate the monoclonal origin of GBMO followed by the development of the astrocytic and oligodendroglial components. The diagnostic determination of the genetic signatures may allow for a better prognostication of the patients.





Similar content being viewed by others
References
S.W. Coons, P.C. Johnson, B.W. Scheithauer, A.J. Yates, D.K. Pearl, Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer 79, 1381–1393 (1997)
C.E. Fuller, R.E. Schmidt, K.A. Roth, P.C. Burger, B.W. Scheithauer, R. Banerjee, K. Trinkaus, R. Lytle, A. Perry, Clinical utility of fluorescence in situ hybridization (FISH) in morphologically ambiguous gliomas with hybrid oligodendroglial/astrocytic features. J. Neuropathol. Exp. Neurol. 62, 1118–1128 (2003)
K. Ueki, R. Nishikawa, Y. Nakazato, T. Hirose, J. Hirato, N. Funada, T. Fujimaki, S. Hojo, O. Kubo, T. Ide, M. Usui, C. Ochiai, S. Ito, H. Takahashi, A. Mukasa, A. Asai, T. Kirino, Correlation of histology and molecular genetic analysis of 1p, 19q, 10q, TP53, EGFR, CDK4, and CDKN2A in 91 astrocytic and oligodendroglial tumors. Clin. Cancer Res. 8, 196–201 (2002)
H. Ohgaki, P. Kleihues, Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J. Neuropathol. Exp. Neurol. 64, 479–489 (2005)
C. Daumas-Duport, B. Scheithauer, J. O’Fallon, P. Kelly, Grading of astrocytomas. A simple and reproducible method. Cancer 62, 2152–2165 (1988)
C.R. Miller, A. Perry, Glioblastoma. Arch. Pathol. Lab. Med. 131, 397–406 (2007)
S.H. Bigner, E. Schrock, Molecular cytogenetics of brain tumors. J. Neuropathol. Exp. Neurol. 56, 1173–1181 (1997)
A. Misra, M. Pellarin, J. Nigro, I. Smirnov, D. Moore, K.R. Lamborn, D. Pinkel, D.G. Albertson, B.G. Feuerstein, Array comparative genomic hybridization identifies genetic subgroups in grade 4 human astrocytoma. Clin. Cancer Res. 11, 2907–2918 (2005)
S. Patt, H. Gries, M. Giraldo, J. Cervos-Navarro, H. Martin, W. Jänisch, J. Brockmöller, p53 gene mutations in human astrocytic brain tumors including pilocytic astrocytomas. Hum. Pathol. 27, 586–589 (1996)
B. Tews, J. Felsberg, C. Hartmann, A. Kunitz, M. Hahn, G. Toedt, K. Neben, L. Hummerich, A. von Deimling, G. Reifenberger, P. Lichter, Identification of novel oligodendroglioma-associated candidate tumor suppressor genes in 1p36 and 19q13 using microarray-based expression profiling. Int. J. Cancer 119, 792–800 (2006)
G. Cairncross, B. Berkey, E. Shaw, R. Jenkins, B. Scheithauer, D. Brachman, J. Buckner, K. Fink, L. Souhami, N. Laperierre, M. Mehta, W. Curran, Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J. Clin. Oncol. 24, 2707–2714 (2006)
J.G. Cairncross, K. Ueki, M.C. Zlatescu, D.K. Lisle, D.M. Finkelstein, R.R. Hammond, J.S. Silver, P.C. Stark, D.R. Macdonald, Y. Ino, D.A. Ramsay, D.N. Louis, Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J. Natl Cancer Inst. 90, 1473–1479 (1998)
J.S. Smith, A. Perry, T.J. Borell, H.K. Lee, J. O’Fallon, S.M. Hosek, D. Kimmel, A. Yates, P.C. Burger, B.W. Scheithauer, R.B. Jenkins, Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J. Clin. Oncol. 18, 636–645 (2000)
M.J. van den Bent, A.F. Carpentier, A.A. Brandes, M. Sanson, M.J.B. Taphoorn, H.J.J.A. Bernsen, M. Frenay, C.C. Tijssen, W. Grisold, L. Sipos, H. Haaxma-Reiche, J.M. Kros, M.C.M. van Kouwenhoven, C.J. Vecht, A. Allgeier, D. Lacombe, T. Gorlia, Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J. Clin. Oncol. 24, 2715–2722 (2006)
J. Felsberg, A. Erkwoh, M.C. Sabel, L. Kirsch, R. Fimmers, B. Blaschke, U. Schlegel, J. Schramm, O.D. Wiestler, G. Reifenberger, Oligodendroglial tumors: refinement of candidate regions on chromosome arm 1p and correlation of 1p/19q status with survival. Brain Pathol. 14, 121–130 (2004)
G. Reifenberger, D.N. Louis, Oligodendroglioma: toward molecular definitions in diagnostic neuro-oncology. J. Neuropathol. Exp. Neurol. 62, 111–126 (2003)
S.H. Bigner, M.R. Matthews, B.K. Rasheed, R.N. Wiltshire, H.S. Friedman, A.H. Friedman, T.T. Stenzel, D.M. Dawes, R.E. McLendon, D.D. Bigner, Molecular genetic aspects of oligodendrogliomas including analysis by comparative genomic hybridization. Am. J. Pathol. 155, 375–386 (1999)
C. Decaestecker, I. Camby, L. Gordower, O. Dewitte, P. Cras, J.J. Martin, J.L. Pasteels, P.V. Ham, J. Brotchi, R. Kiss, I. Salmon, Characterization of astroglial versus oligodendroglial phenotypes in glioblastomas by means of quantitative morphonuclear variables generated by computer-assisted microscopy. J. Neuropathol. Exp. Neurol. 57, 791–802 (1998)
J. He, K. Mokhtari, M. Sanson, Y. Marie, M. Kujas, S. Huguet, P. Leuraud, L. Capelle, J.Y. Delattre, J. Poirier, K. Hoang-Xuan, Glioblastomas with an oligodendroglial component: a pathological and molecular study. J. Neuropathol. Exp. Neurol. 60, 863–871 (2001)
J.A. Kraus, K. Lamszus, N. Glesmann, M. Beck, M. Wolter, M. Sabel, D. Krex, T. Klockgether, G. Reifenberger, U. Schlegel, Molecular genetic alterations in glioblastomas with oligodendroglial component. Acta Neuropathol (Berl) 101, 311–320 (2001)
J.W. Jeuken, S.H. Sprenger, R.H. Boerman, A. von Deimling, H.L. Teepen, J.J. van Overbeeke, P. Wesseling, Subtyping of oligo-astrocytic tumours by comparative genomic hybridization. J. Pathol. 194, 81–87 (2001)
C. Walker, D.G. du Plessis, K.A. Joyce, Y. Machell, J. Thomson-Hehir, S.A.A. Haddad, J.C. Broome, P.C. Warnke, Phenotype versus genotype in gliomas displaying inter- or intratumoral histological heterogeneity. Clin. Cancer Res. 9, 4841–4851 (2003)
D.A. Hilton, M. Penney, L. Pobereskin, H. Sanders, S. Love, Histological indicators of prognosis in glioblastomas: retinoblastoma protein expression and oligodendroglial differentiation indicate improved survival. Histopathology 44, 555–560 (2004)
P. Kleihues, P.C. Burger, K.D. Aldape, D.J. Brat, W. Biernat, D.D. Bigner, Y. Nakazato, K.H. Plate, F. Giangaspero, A. von Deimling, H. Ohgaki, W.K. Cavenee, Glioblastoma, in WHO Classification of Tumours of the Central Nervous System, ed. by D.N. Louis, H. Ohgaki, O.D. Wiestler, W.K. Cavenee (International Agency for Research on Cancer (IARC) Press, Lyon, 2007), pp. 33–49
P. Kleihues, W.K. Cavenee, Pathology and Genetics of Tumours of the Nervous System, World Health Organization Classification of Tumours (International Agency for Research on Cancer (IARC) Press, Lyon, 2000)
R. Koschny, T. Koschny, U.G. Froster, W. Krupp, M.A. Zuber, Comparative genomic hybridization in glioma: a meta-analysis of 509 cases. Cancer Genet. Cytogenet. 135, 147–159 (2002)
E. Schrock, C. Blume, M.C. Meffert, S. du Manoir, W. Bersch, M. Kiessling, T. Lozanowa, G. Thiel, R. Witkowski, T. Ried, T. Cremer, Recurrent gain of chromosome arm 7q in low-grade astrocytic tumors studied by comparative genomic hybridization. Genes Chromosom. Cancer 15, 199–205 (1996)
C. Lengauer, I. Dunham, T. Featherstone, T. Cremer, Generation of alphoid DNA probes for fluorescence in situ hybridization (FISH) using the polymerase chain reaction. Methods Mol. Biol. 33, 51–61 (1994)
E. Schrock, G. Thiel, T. Lozanova, S. du Manoir, M.C. Meffert, A. Jauch, M.R. Speicher, P. Nürnberg, S. Vogel, W. Jänisch, Comparative genomic hybridization of human malignant gliomas reveals multiple amplification sites and nonrandom chromosomal gains and losses. Am. J. Pathol. 144, 1203–1218 (1994)
N. Aldosari, R.N. Wiltshire, A. Dutra, E. Schrock, R.E. McLendon, H.S. Friedman, D.D. Bigner, S.H. Bigner, Comprehensive molecular cytogenetic investigation of chromosomal abnormalities in human medulloblastoma cell lines and xenograft. Neuro. Oncol. 4, 75–85 (2002)
P.H. Wessels, A. Twijnstra, A.G.H. Kessels, B. Krijne-Kubat, P.H. Theunissen, M.I.J. Ummelen, F.C.S. Ramaekers, A.H. Hopman, Gain of chromosome 7, as detected by in situ hybridization, strongly correlates with shorter survival in astrocytoma grade 2. Genes Chromosom. Cancer 33, 279–284 (2002)
K.S. Reddy, Assessment of 1p/19q deletions by fluorescence in situ hybridization in gliomas. Cancer Genet. Cytogenet. 184, 77–86 (2008)
T. Nagasaka, M. Gunji, N. Hosokai, K. Hayashi, H. Ikeda, M. Ito, S. Inao, FISH 1p/19q deletion/imbalance for molecular subclassification of glioblastoma. Brain Tumor Pathol. 24, 1–5 (2007)
C.A. Klein, O. Schmidt-Kittler, J.A. Schardt, K. Pantel, M.R. Speicher, G. Riethmüller, Comparative genomic hybridization, loss of heterozygosity, and DNA sequence analysis of single cells. Proc. Natl Acad. Sci. U.S.A. 96, 4494–4499 (1999)
W. Paulus, J. Peiffer, Intratumoral histologic heterogeneity of gliomas. A quantitative study. Cancer 64, 442–447 (1989)
D.N. Louis, H. Ohgaki, O.D. Wiestler, W.K. Cavenee, P.C. Burger, A. Jouvet, B.W. Scheithauer, P. Kleihues, The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 114, 97–109 (2007)
Y. Nakazato, K.H. Plate, F. Giangaspero, A. von Deimling, H. Ohgaki, W.K. Cavenee, Glioblastoma, in WHO Classification of Tumours of the Nervous System, ed. by D.N. Louis, H. Ohgaki, O.D. Wiestler, W.K. Cavenee (IARC, Lyon, 2007), pp. 33–49
J.E. Bromberg, M.J. van den Bent, Oligodendrogliomas: Molecular biology and treatment. Oncologist 14, 155–163 (2009)
T. Nishizaki, S. Ozaki, K. Harada, H. Ito, H. Arai, T. Beppu, K. Sasaki, Investigation of genetic alterations associated with the grade of astrocytic tumor by comparative genomic hybridization. Genes Chromosom. Cancer 21, 340–346 (1998)
A. von Deimling, D.N. Louis, K. von Ammon, I. Petersen, T. Hoell, R.Y. Chung, R.L. Martuza, D.A. Schoenfeld, M.G. Yasargil, O.D. Wiestler, Association of epidermal growth factor receptor gene amplification with loss of chromosome 10 in human glioblastoma multiforme. J. Neurosurg. 77, 295–301 (1992)
C.A. Griffin, P. Burger, L. Morsberger, R. Yonescu, S. Swierczynski, J.D. Weingart, K.M. Murphy, Identification of der(1;19)(q10;p10) in five oligodendrogliomas suggests mechanism of concurrent 1p and 19q loss. J. Neuropathol. Exp. Neurol. 65, 988–994 (2006)
R.B. Jenkins, H. Blair, K.V. Ballman, C. Giannini, R.M. Arusell, M. Law, H. Flynn, S. Passe, S. Felten, P.D. Brown, E.G. Shaw, J.C. Buckner, A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 66, 9852–9861 (2006)
K. Ichimura, A.P. Vogazianou, L. Liu, D.M. Pearson, L.M. Backlund, K. Plant, K. Baird, C.F. Langford, S.G. Gregory, V.P. Collins, 1p36 is a preferential target of chromosome 1 deletions in astrocytic tumours and homozygously deleted in a subset of glioblastomas. Oncogene 27, 2097–2108 (2008)
A. Idbaih, Y. Marie, G. Pierron, C. Brennetot, K. Hoang-Xuan, M. Kujas, K. Mokhtari, M. Sanson, J. Lejeune, A. Aurias, O. Delattre, J.Y. Delattre, Two types of chromosome 1p losses with opposite significance in gliomas. Ann. Neurol. 58, 483–487 (2005)
A. Korshunov, R. Sycheva, A. Golanov, The prognostic relevance of molecular alterations in glioblastomas for patients age <50 years. Cancer 104, 825–832 (2005)
H. Ohgaki, P. Kleihues, Genetic pathways to primary and secondary glioblastoma. Am. J. Pathol. 170, 1445–1453 (2007)
M.C. Schmidt, S. Antweiler, N. Urban, W. Mueller, A. Kuklik, B. Meyer-Puttlitz, O.D. Wiestler, D.N. Louis, R. Fimmers, A. von Deimling, Impact of genotype and morphology on the prognosis of glioblastoma. J. Neuropathol. Exp. Neurol. 61, 321–328 (2002)
T.C.G.A.R. Network, Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455, 1061–1068 (2008)
T. Homma, T. Fukushima, S. Vaccarella, Y. Yonekawa, P.L.D. Patre, S. Franceschi, H. Ohgaki, Correlation among pathology, genotype, and patient outcomes in glioblastoma. J. Neuropathol. Exp. Neurol. 65, 846–854 (2006)
M. Salvati, A.I. Formichella, A. D’Elia, C. Brogna, A. Frati, F. Giangaspero, R. Delfini, A. Santoro, Cerebral glioblastoma with oligodendrogliomal component: analysis of 36 cases. J. Neurooncol. 94, 129–134 (2009)
L.W. Pinto, M.B. Araujo, A.L. Vettore, L. Wernersbach, A.C. Leite, L.M. Chimelli, F.A. Soares, Glioblastomas: correlation between oligodendroglial components, genetic abnormalities, and prognosis. Virchows Arch. 452, 481–490 (2008)
J.A. Kraus, J. Koopmann, P. Kaskel, D. Maintz, S. Brandner, J. Schramm, D.N. Louis, O.D. Wiestler, A. von Deimling, Shared allelic losses on chromosomes 1p and 19q suggest a common origin of oligodendroglioma and oligoastrocytoma. J. Neuropathol. Exp. Neurol. 54, 91–95 (1995)
M. Qu, T. Olofsson, S. Sigurdardottir, C. You, H. Kalimo, M. Nister, A. Smits, Z.P. Ren, Genetically distinct astrocytic and oligodendroglial components in oligoastrocytomas. Acta Neuropathol. 113, 129–136 (2007)
Z.-Q. Dong, J.C.-S. Pang, C.Y.-K. Tong, L.-F. Zhou, H.-K. Ng, Clonality of oligoastrocytomas. Hum. Pathol. 33, 528–535 (2002)
D. Trost, M. Ehrler, R. Fimmers, J. Felsberg, M.C. Sabel, L. Kirsch, J. Schramm, O.D. Wiestler, G. Reifenberger, R.G. Weber, Identification of genomic aberrations associated with shorter overall survival in patients with oligodendroglial tumors. Int. J. Cancer 120, 2368–2376 (2007)
K.B. Fallon, C.A. Palmer, K.A. Roth, L.B. Nabors, W. Wang, M. Carpenter, R. Banerjee, P. Forsyth, K. Rich, A. Perry, Prognostic value of 1p, 19q, 9p, 10q, and EGFR-FISH analyses in recurrent oligodendrogliomas. J. Neuropathol. Exp. Neurol. 63, 314–322 (2004)
M. Nakamura, F. Yang, H. Fujisawa, Y. Yonekawa, P. Kleihues, H. Ohgaki, Loss of heterozygosity on chromosome 19 in secondary glioblastomas. J. Neuropathol. Exp. Neurol. 59, 539–543 (2000)
R.G. Weber, M. Sabel, J. Reifenberger, C. Sommer, J. Oberstrass, G. Reifenberger, M. Kiessling, T. Cremer, Characterization of genomic alterations associated with glioma progression by comparative genomic hybridization. Oncogene 13, 983–994 (1996)
E.C. Wooten, D. Fults, R. Duggirala, K. Williams, A.P. Kyritsis, M.L. Bondy, V.A. Levin, P. O’Connell, A study of loss of heterozygosity at 70 loci in anaplastic astrocytoma and glioblastoma multiforme with implications for tumor evolution. Neuro. Oncol. 1, 169–176 (1999)
Acknowledgement
This work was supported by a research grant from the Interdisziplinäres Zentrum für Klinische Forschung (IZKF) at the Medizinische Fakultät der Friedrich-Schiller-Universität Jena, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is a reprint from ‘Glioblastomas with oligodendroglial component-common origin of the different histological parts and genetic subclassification, Barbara Klink, Ben Schlingelhof, Martin Klink, Karen Stout-Weider, Stephan Patt, Evelin Schrock’ originally published in Analytical Cellular Pathology/Cellular Oncology, Volume 33, number 1, 2010, pp. 37–54, IOS Press.
An erratum to this article can be found at http://dx.doi.org/10.1007/s13402-011-0055-3
Rights and permissions
About this article
Cite this article
Klink, B., Schlingelhof, B., Klink, M. et al. Glioblastomas with oligodendroglial component-common origin of the different histological parts and genetic subclassification. Cell Oncol. 34, 261–275 (2011). https://doi.org/10.1007/s13402-011-0034-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-011-0034-8