Abstract
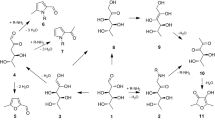
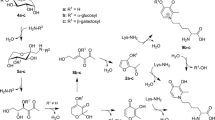
The behavior of different lysine Amadori compounds during acid hydrolysis was investigated in order to determine the molar yield of furosine and the other hydrolysis products. Based on this, the conversion factors for calculating the content of Amadori compound and lysine modification before hydrolysis can be derived. For that purpose, the peptide-bound Amadori compounds Nε-(1-deoxy-D-fructosyl)-, Nε-(1-deoxy-D-tagatosyl)-, Nε-(1-deoxy-D-lactulosyl)- and Nε-(1-deoxy-D-maltulosyl)hippuryllysine as well as free Nε-(1-deoxy-D-fructosyl)lysine were synthesized. For isolation of peptide-bound Amadori compounds, an optimized enzyme-enhanced reversed phase-high pressure liquid chromatography procedure was developed. Pyridosine and Nε-(carboxymethyl)hippuryllysine were synthesized as reference materials. After acid hydrolysis with 6 M hydrochloric acid the, molar yields of furosine were determined to be 32% for fructosyllysine, 34% for lactulosyl- and maltulosyllysine and 42% for tagatosyllysine. Hydrolysis with 8 M hydrochloric acid resulted in a higher yield of furosine for Amadori compounds containing a fructosyl-moiety, 46% for fructosyllysine, 50% for lactulosyllysine and 51% for maltulosyllysine. Compared with this, the molar yield for furosine was not affected by concentration of hydrochloric acid in the case of tagatosyllysine. Based on these conversion factors a reliable calculation of the amount of Amadori compound or lysine modification and with it the evaluation of the progress of the "early" Maillard reaction in foods and biological samples is possible via the quantification of lysine and furosine after acid hydrolysis.





Similar content being viewed by others
References
Resmini P, Pellegrino L, Battelli G (1990) Ital J Food Sci 3:173–183
Italian Regulation of 15 Dezember 2000 (2000) Gazetta Ufficiale RI No. 31 07.02.2001
Commission Regulation (EC) No 2527/98 of 25 November 1998 (1998) Official Journal of the European Communities L 317 , 26.11.1998, pp. 14–18
Pompei C, Spagnolello A (1997) Meat Sci 46:139–146
Guerra–Hernandez E, Corzo N, Garcia–Villanova B (1998) J Cereal Sci 29:171–176
Villamiel M, del Castillo MD, Corzo N, Olano A (2001) J Sci Food Agric 81:790–793
Marconi E, Caboni MF, Messia MC, Panfili G (2002) J Agric Food Chem 50:2825–2829
Rada-Mendoza M, Olano A, Villamiel, M (2002) J Agric Food Chem 50:4141–4145
Floridi A, Trizza V, Paolotti, P, Lucarrelli, C (1999) J Chromatogr A 846:65–71
Wu YC, Monnier V, Friedlander M (1995) J Chromatogr B 667:328–332
Brandt A, Erbersdobler HF (1973) Landwirtsch Forsch Sonderh 28:115–119
Finot PA, Mauron J (1972) Helv Chim Acta 55:1153–1164
Finot PA, Bujard E, Mottu F (1977) In: Friedman M (ed) Protein crosslinking. Nutritional and medical consequences, Plenum Press, New York, pp 343–365
Steinig J, Monatag A (1982) Z Lebensm Unters Forsch 174:453–457
Szölgyenyi GP, Winsauer KJB, Deutsch B (1989) Monatshefte für Chemie/Chemical Monthly 120:1147–1158
Van Boekel MAJS (1998) Food Chem 62:403–414
Reutter M, Eichner K (1989) Z Lebensm Unters Forsch 188:28–35
Finot PA, Mauron J (1969) Helv Chim Acta 52:1488–1495
Henle T, Walter AW, Klostermeyer H (1994) Z Lebensm Unters Forsch 198:66–67
Ikeda K, Higashi T, Sano H, Jinnouchi Y, Yoshida M, Araki T, Ueda S, Horiuchi S (1996) Biochemistry 35:8075–8083
Henle T, Walter H, Krause I, Klostermeyer H (1991) Int Dairy J 1:125–135
Smith PR, Thornalley PJ (1992) Carbohydr Res 223:293–298
Ambler, RP (1972) Methods Enzymol 25:143–154
Finot PA, Deutsch R, Bujard E (1981) In: Erikson C (ed) Progr. Food Nutr. Sci. vol. 5, Oxford:Pergamon Press, pp. 345–355
Henle T, Zehtner G, Klostermeyer H (1995) Z Lebensm Unters Forsch 200:235–237
Möller AB, Andrews AT, Cheeseman GC (1977) J Dairy Res 44:267–275
Ahmed MU, Thorpe SR, Baynes JW (1986) J Biol Chem 261:4889–4894
Acknowledgements
We thank Karla Schlosser and Dr. Horst Nötzold, Institute of Food Chemistry, Technical University of Dresden, for performing the amino acid analyses and Ralf Preuss and Steffen Seifert, Institute of Food Chemistry, Technical University of Dresden, for synthesizing Nε-(carboxymethyl)hippuryllysine. We are grateful to the members of the Institute of Organic Chemistry, Technical University of Dresden, Dr. Dieter Scheller and Annett Rudolf for recording the NMR spectra and the valuable help to interpret them, Dr. Herbert Kroschwitz for acquisition of ESI-MS data and Anke Pertitz for performing the elemental analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krause, R., Knoll, K. & Henle, T. Studies on the formation of furosine and pyridosine during acid hydrolysis of different Amadori products of lysine. Eur Food Res Technol 216, 277–283 (2003). https://doi.org/10.1007/s00217-002-0649-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-002-0649-0