Abstract
Background
Fluorescence-guided surgery (FGS) is a technique used to enhance visualization of tumor margins in order to increase the extent of tumor resection in glioma surgery. In this paper, we systematically review all clinically tested fluorescent agents for application in FGS for glioma and all preclinically tested agents with the potential for FGS for glioma.
Methods
We searched the PubMed and Embase databases for all potentially relevant studies through March 2016. We assessed fluorescent agents by the following outcomes: rate of gross total resection (GTR), overall and progression-free survival, sensitivity and specificity in discriminating tumor and healthy brain tissue, tumor-to-normal ratio of fluorescent signal, and incidence of adverse events.
Results
The search strategy resulted in 2155 articles that were screened by titles and abstracts. After full-text screening, 105 articles fulfilled the inclusion criteria evaluating the following fluorescent agents: 5-aminolevulinic acid (5-ALA) (44 studies, including three randomized control trials), fluorescein (11), indocyanine green (five), hypericin (two), 5-aminofluorescein-human serum albumin (one), endogenous fluorophores (nine) and fluorescent agents in a pre-clinical testing phase (30). Three meta-analyses were also identified.
Conclusions
5-ALA is the only fluorescent agent that has been tested in a randomized controlled trial and results in an improvement of GTR and progression-free survival in high-grade gliomas. Observational cohort studies and case series suggest similar outcomes for FGS using fluorescein. Molecular targeting agents (e.g., fluorophore/nanoparticle labeled with anti-EGFR antibodies) are still in the pre-clinical phase, but offer promising results and may be valuable future alternatives.
Similar content being viewed by others
Introduction
Radical surgical resection is the surgical treatment of choice for gliomas [95, 102]. Balancing maximum cytoreduction with preservation of healthy brain tissue is complicated by the infiltrative nature of these tumors [88, 96]. Fluorescent agents are increasingly being tested and used to distinguish tumor from normal parenchyma thus improving surgical resection while sparing healthy brain tissue [17, 57, 59, 76, 119]. The only fluorescent agent that has been tested in a multi-center randomized controlled trial (RCT) and the only agent currently approved for resection of high-grade gliomas (HGGs) in Europe, Canada, and Japan is 5-aminolevulinic acid (5-ALA) [67]. In clinical studies, the use of 5-ALA for fluorescence-guided surgery (FGS) has been shown to increase the rate of gross-total resection (GTR) and the length of progression-free survival (PFS) [99]. As a relatively nascent innovation, FGS for glioma is still limited by many factors, which depend on the fluorescent agent used. In this systematic review, we assess the use of all clinically tested fluorescent agents in FGS for glioma. Furthermore, we evaluate all pre-clinically tested fluorescent agents with the potential for FGS for glioma.
Methods
Search strategy
We performed an extended search in PubMed and Embase databases according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines on March 21, 2016. We included all articles investigating the use of fluorescent agents for identification or resection of glioma tumor cells in both the clinical and pre-clinical settings. This review is restricted to published literature. Only papers written in English and Dutch were included. The search was not limited by date of publication. We did not include pre-clinical studies on 5-ALA and fluorescein, as these agents have been used extensively in the clinical setting. The search syntax is available in Table 1. The systematic search was complemented by additional citations identified by hand searching the bibliographies of the papers retrieved by the electronic search. The title and abstracts of retrieved studies were screened, and full texts of potentially suitable articles were read by three authors (JS, RS, IM). Disagreements were resolved by discussion.
Data extraction
The following data were extracted from selected papers: year of publication, name of first author, fluorescent agent tested, study design, number of patients, tumor grade, GTR rate, sensitivity and specificity of the fluorescent agent for tumor tissue, tumor-to-normal ratio (TNR) of the fluorescent signal, median survival, progression-free survival (PFS), and incidence of adverse events. GTR was defined as no residual enhancement on post-operative magnetic resonance imaging (MRI). Overall survival and PFS was quantified in months. Among the included studies, histological accuracy was quantified in two ways. Some studies collected tissue samples near the tumor margin from fluorescent and non-fluorescent areas for histopathological examination and calculated the sensitivity and specificity of distinguishing tumor from healthy brain tissue. Others measured the fluorescent signal intensity from tumor and brain tissue and calculated a TNR. We considered grade I and II tumors as low-grade gliomas (LGGs) and grade III and IV gliomas as high-grade gliomas (HGGs) according to the 2016 World Health Organization (WHO) classification of tumors of the central nervous system [60].
Results
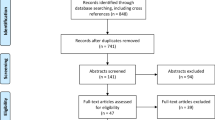
We identified 2155 studies in PubMed and Embase after duplicates were removed. After screening by title and abstract, 237 studies remained for full-text review. Of these, we included 105 studies describing the use of clinically or pre-clinically tested fluorescent agents for application in FGS for glioma (Fig. 1). Detailed characteristics of all 105 studies included in this review are available in Table 2. Three studies were randomized clinical trials, of which two had partially the same data set. Three studies were meta-analyses. The other clinical studies were retrospective or prospective cohort studies, or case series. Preclinical studies included human or animal ex vivo studies, animal in vivo studies, or in vitro studies.
Clinically tested fluorescent agents
Sixty-four studies describe the clinical use of fluorescent agents [1, 3, 5, 6, 9, 11, 13–16, 22–26, 28–31, 33–35, 37, 38, 40–42, 44, 49, 52–54, 58, 64–66, 69, 71, 74, 75, 78, 80–82, 84–87, 89, 92, 94, 97–101, 105–107, 109, 110, 113, 115, 118]. Three ways of labeling tumor cells were identified in the literature: (1) passive, (2) metabolic, and (3) molecular labeling. Passive labeling occurs when enhanced permeability and retention allow exogenous agents to accumulate at the tumor site. The damaged blood–brain barrier (BBB) allows exogenous agents (e.g., fluorescein or ICG) to concentrate in glioma tissue [67]. Metabolic fluorescent agents (e.g., 5-ALA) are internalized and metabolized intracellularly [99]. Molecular targeting refers to the binding of agents to specific molecules on the cell surface of the tumor cell. A popular target is the epidermal growth factor receptor (EGFR) [67].
5-aminolevulinic acid (5-ALA)
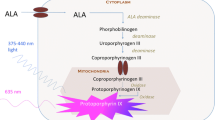
5-ALA is a metabolic targeting agent and the natural precursor of the fluorescent protoporphyrin (PpIX) in the heme synthesis pathway. Ferrochelatase converts PpIX into heme intracellularly by adding a Fe2 + −ion. In glioma cells, ferrochelatase is downregulated. Therefore, these cells accumulate PpIX to a fluorescently detectable level when this pathway is overloaded with exogenous 5-ALA. PpIX absorbs light between 375 and 440 nm and emits light between 640 and 710 nm [119].
Forty-four clinical studies described the use of 5-ALA for glioma surgery [3, 5, 6, 13–16, 22, 24–26, 28, 30, 31, 33, 34, 37, 40–42, 44, 66, 69, 74, 75, 78, 81, 82, 84, 85, 87, 89, 94, 97–101, 105, 106, 109, 110, 113, 115], of which only six studies included LGGs [28, 33, 84, 109, 110, 113]. 5-ALA is the only fluorescent agent that has been tested in an RCT. Three RCTs compared FGS with 5-ALA to surgery without fluorescent guidance, with two studies including partially the same patients [26, 99, 101]. The study by Stummer et al. showed a higher GTR rate for the 5-ALA group compared to the group that was operated without 5-ALA (65 vs. 35%) [99]. In observational studies, the rate of GTR after 5-ALA administration ranges from 25 to 94.3% for HGGs [3, 5, 6, 14, 16, 22, 24, 25, 30, 31, 34, 40, 42, 44, 69, 75, 82, 87, 89, 94, 98, 99, 105, 106, 113]. However, studies that included a control group all confirm a significantly higher GTR rate in the FGS group [14, 24, 82, 94, 99]. Two RCTs reported an increased PFS (8.6 vs. 4.8 months) [26] or increased rate of 6-month PFS (46 vs. 28%) [101]. Regarding extending overall survival by using 5-ALA during neurosurgical resection, results vary between non-significant (14–15 vs. 13–14 months) [99, 101] and significant (12 vs. 6 months) [26] survival benefits.
Observational studies show a broad range regarding sensitivity and specificity in discriminating HGG tissue from healthy brain tissue [13, 25, 28, 33, 34, 37, 40, 41, 74, 81, 98, 105, 110, 113, 115]. For discriminating glioblastoma multiforme (GBM) tissue from healthy brain tissue, the sensitivity and specificity ranged from 70 to 95% and 43 to 100%, respectively [25, 34, 74, 98]. All four studies that included both LGG and HGG patients reported a lower sensitivity and specificity in LGGs [28, 33, 110, 113]. To increase the accuracy of LGGs, FGS was combined with intra-operative confocal microscopy [84] or an intraoperative probe for quantitative fluorescence measurement [109]. Other intraoperative techniques used to increase the extent of glioma resection are photodynamic therapy (PDT) [26], iMRI [14, 30, 34, 40, 78, 85], intra-operative CT [5], exoscope imaging [6, 75], fluorescence spectrometry [37, 100], confocal microscopy [84], and intraoperative mapping [22, 89].
Three meta-analyses have been performed to evaluate the literature on 5-ALA [27, 103, 120]. GTR and PFS were improved in all meta-analyses that compared 5-ALA with conventional white-light surgery. A significant difference in overall survival was reported in two meta-analyses [27, 120]. One meta-analysis reported no significant difference in overall survival [103], however, this meta-analysis also included studies on fluorescein for overall survival. The mean sensitivity and specificity in distinguishing tumor from healthy brain tissue ranged between 83 and 87% and 89 and 91% in all three meta-analyses, respectively.
Only the RCT by Stummer et al. 2011 found a significant difference in the incidence of adverse effects. The 5-ALA group had more frequent deterioration at the National Institute of Health Stroke Scale (NIH-SS) at 48 h after surgery [101]. Other reported adverse effects of 5-ALA include nausea, mild hypotension, elevated liver enzymes, and photosensitivity up to 48 h post administration [12, 119].
Fluorescein
Eleven papers described the use of fluorescein as a fluorescent agent in glioma surgery [1, 11, 23, 38, 52, 54, 64, 65, 71, 86, 92]. All were observational studies including patients with HGG. Only three studies included patients with LGG [11, 64, 86]. Fluorescein is a passive targeting agent commonly used for ophthalmic examinations of the retina [67]. Interestingly, as early as in 1948, a study demonstrated a positive predictive value of 96% in locating brain tumors [65]. Fluorescein is administered intravenously at induction of anesthesia or at time of opening the dura. It is excited at a wavelength of 460–500 nm and has an emission spectral range of 540–690 nm. As this is within the spectrum of visible light, fluorescein is used with [1, 23, 27, 38, 54, 71, 86, 120] or without a filter on the surgical microscope [11, 52, 64, 65, 92].
Nine studies showed that upon administration of fluorescein, GTR can be achieved in 79–84% of patients [1, 11, 23, 38, 52, 54, 71, 86, 92]. Studies comparing the use of fluorescein to conventional white light surgery showed a GTR-rate of 30–55% in the latter group [11, 52, 92]. The use of a special filter integrated into the microscope resulted in an even higher GTR rate of 80–100%; this integrated filter allowed for more accurate delineation at the tumor border and required less fluorescein for visualization (3–8 mg/kg with filter instead of 20 mg/kg without filter in the microscope) [1, 23, 27, 38, 54, 71, 86, 120].
The effect of fluorescein on survival has been evaluated by four groups. Chen et al. found an increase in PFS (7.4 vs. 5.4 months) [11]. Others did not find an increase in overall survival [52, 92] or did not compare with a control group [1].
Three papers reported on the presence of tumor cells in fluorescein negative areas [11, 54, 92]. Others reported that fluorescein identifies tumor tissue with a sensitivity and specificity of 82–94% and 90–91%, respectively [1, 23, 64]. To enhance histological accuracy, Martirosyan et al. explored the use of confocal microscopy in combination with fluorescein [64]. This technique makes use of a handheld probe containing a miniature scanner. The scanner can be placed in direct contact with the tissue of interest and can be visualized on a connected external monitor. The imaging field has a diameter of 0.5 mm. With the integrated depth actuator in the probe, the surgeon can focus on a specific depth beneath the contact plane ranging from 0 to 500 μm. Confocal microscopy with fluorescein is able to visualize individual invading cells at the tumor margin and even subcellular histological features. A sensitivity and specificity of 91 and 94%, respectively, was reported in distinguishing tumor from healthy brain tissue [64].
Studies that included patients with LGG did not stratify for tumor grade. One study reported that visualization was less obvious in LGGs or in recurrent tumors (that had previously been resected or irradiated), due to accumulation of scar tissue. In a survey of five neurosurgeons, fluorescein was rated as ‘helpful’ in visualizing gliomas in 80% of the cases [86].
Side effects of fluorescein include yellow coloration of skin, mucosa, and urine up to 24 h after surgery, generally seen only after high-dose (20 mg/kg) fluorescein [65, 71, 92]. No side effects were detected with low-dose (2–8 mg/kg) fluorescein [1, 23, 38, 54, 86]. Anaphylactic reactions to fluorescein have been reported [117].
Indocyanine green (ICG)
Two clinical and three pre-clinical studies reported on the use of ICG for glioma surgery [29, 35, 36, 39, 63, 93]. ICG has a peak emission at 820 nm. This near-infrared (NIR) spectrum allows visualization of deeper structures than does visible wavelength. ICG works as a passive targeting agent and depends on the breakdown of the BBB to concentrate at the tumor site. It is already used for several clinical applications, including determining cardiac output, ascertaining hepatic function and liver blood flow, and implementing ophthalmic angiography. ICG is administered intravenously before resection or afterwards to visualize remaining tumor tissue [67].
No articles evaluated the rate of GTR or survival in patients treated with ICG. In rat glioma models, ICG shows an underestimation of 1 mm of the histological tumor border [39] and a sensitivity and specificity of 90 and 93%, respectively [36]. In humans, low-dose ICG (1–2 mg/kg) combined with a filter microscope revealed remaining tumor tissue after resection. Detection was superior in high-grade compared to low-grade gliomas [35]. In a recent case series that combined both fluorescent agents for GBM resection, three tumor zones could be distinguished from the center to the margin of the tumor: a central zone that was stained by both compounds, a zone that was stained by only ICG and not 5-ALA, and the most peripheral zone that contained tumor cells but was not stained by any of the compounds. This suggests that ICG is superior to 5-ALA in staining tumor tissue with a low cell density [29]. Confocal microscopy visualized individual invading tumor cells in peritumoral tissue in a GBM mouse model, and subcellular structures correlated with histological features. The NIR wavelength allowed an imaging plan depth of >350 μm [63].
No complications or adverse effects of ICG were mentioned in these studies. Anaphylactic reactions to ICG have been reported [72].
5-aminoflurescein human serum albumin
One case series assessed the passive tumor-targeting agent 5-aminofluorescein (AFL) labeled to human serum albumin (HSA) (excitation 495 nm, emission 535 nm). FGS with AFL-HSA in 13 patients with HGG resulted in a GTR rate of 69%. No phototoxic, allergic, or other side effects related to AFL-HSA were observed [53].
Hypericin
One case series and one pre-clinical study assessed hypericin, a passive tumor-targeting agent. Hypericin (excitation 415–495 nm; emission 590–650 nm) is intravenously administered in patients undergoing surgery for HGG. Tissue samples from fluorescent and non-fluorescent areas showed a sensitivity and specificity in distinguishing human brain and tumor tissue of 91–94% and 90–100%, respectively. No side effects were observed [80]. In an animal study, rats were implanted with GBM cells and intravenously injected with hypericin. The accumulation of hypericin in the brain was studied ex vivo under a fluorescence microscope. The tumor-to-normal ratio (TNR) was 19.8, after correction for auto-fluorescence [70]. No adverse effects were observed.
Endogenous fluorophores
Endogenous fluorophores (e.g., NAD(P) H, FAD, and collagen) in brain and tumor tissue can emit fluorescent signals after excitation. Nine studies, five of which were clinical, assessed the use of endogenous fluorophores [9, 49, 50, 56, 58, 62, 79, 107, 118]. Four case series evaluated endogenous fluorophores by using optical spectroscopy [9, 58, 107, 118] and one case series used multiphoton excitation tomography [49]. With optical spectroscopy, a fiber optic probe is placed against the tissue of interest to detect the fluorescent signal. An algorithm then distinguishes brain and tumor tissue [9]. Two studies including both patients with HGG and with LGG achieved a sensitivity and specificity in discriminating infiltrative tumor margin and healthy tissue of 94–100% and 76–93%, respectively [58, 107]. The decrease of fluorescent signal in time provides additional information. Adding this extra dimension to the algorithm, sensitivity and specificity in discriminating LGG from normal brain tissue were 90–100% and 98–100%, respectively. Due to necrosis and a high degree of heterogeneity, however, the sensitivity and specificity for HGG were 47–95% and 94–96%, respectively [9, 118].
Multiple excitation beams from different angles allow excitation wavelengths to be in the infrared spectrum. This reduces phototoxicity, light scattering, and artifacts from blood, and increases the penetration depth. Excitation only occurs when two low-energy photons are simultaneously absorbed by the fluorophore where the laser beams coincide, reducing the amount of background signal. Kantelhardt et al. were the first to use multiphoton excitation tomography intra-operatively in humans, and reported the ability to differentiate between tumor and brain tissue on cellular and subcellular levels [49]. No adverse effects were observed.
Pre-clinically tested fluorescent agents
Thirty studies described the results of fluorescent agents in a pre-clinical phase (Table 2) [2, 4, 7, 8, 10, 18–21, 32, 43, 45–48, 51, 55, 61, 68, 73, 77, 83, 90, 91, 104, 108, 111, 112, 116, 121]. Within this group of fluorescent agents, a broad distinction could be made between molecular fluorophores and nanoparticles. Molecular fluorophores are small-sized molecules with fluorescent properties. ICG and fluorescein are examples of clinically tested organic molecular fluorophores [76]. Nanoparticles are structures of nanometer size (1–100 nm). Depending on their structure, nanoparticles can contain optical properties or obtain optical properties by labeling with fluorophores. Targeting properties of both fluorophores and nanoparticles are tunable by adding targeting peptides [76]. Due to their larger size, nanoparticles are often less susceptible to nonspecific binding than molecular fluorophores. This nonspecific binding can modify the optical properties of the fluorophore and the function of cellular proteins [114]. In this review, we will discuss the pre-clinically tested fluorescent agents according to this distinction. We will discuss nanoparticles and fluorophores bound to epidermal growth factor receptor (EGFR) targeting peptides in a separate section.
Pre-clinically, 18 studies evaluated molecular fluorophores [2, 4, 7, 18, 20, 21, 32, 43, 45, 55, 61, 77, 91, 104, 111, 112, 116] and 12 studies evaluated nanoparticles [10, 19, 46–48, 51, 68, 73, 83, 90, 108, 121]. Four of these 30 studies evaluated fluorophores or nanoparticles bound to EGF or anti-EGFR antibodies [21, 48, 90, 91]. Other fluorophores included IRDye 800CW-RGD [43], Cy5-SBK2 [7], Cy3-AS1411-TGN [61], cyclic-RGD-PLGC (Me) AG-ACPP [18], CH1055 [4], CLR1502 [104], anti-TRP-2 labeled with Alexa fluor 488 or 750 [32], motexafin gadolinium [77], BLZ-100 [8], Angiopep-2-Cy5.5 [116], DA364-Cy5.5 [55], PARPi-Fl [45], chlorotoxin:Cy5.5 [111], PEG-Cy5.5 [2], GB119-Cy5 [20] and cetuximab-IRDye 800CW [112]. Other nanoparticles included quantum dots [10, 46], iron oxide nanoparticles [51, 108, 121], polymer based nanoparticles [19, 47, 73], upconversion nanoparticles (UCNPs) [68], and liposomal nanocarriers [83].
Molecular fluorophores
Eighteen papers described molecular fluorophores with molecular (15), metabolic (one), and passive (two) targeting mechanisms [2, 4, 7, 8, 18, 20, 21, 32, 43, 45, 55, 61, 77, 91, 104, 111, 112, 116]. Fluorophores conjugated to the integrin-targeting peptide RGD (IRDye 800CW-RGD) [43] or the protein tyrosine phosphatase mu-targeting peptide SBK2 (Cy5-SBK2) [7] showed a TNR of 16.3–79.7 and 11.7–19.8, respectively, dependent on the glioma cell line being observed. Cy5-SBK2 was tested in vivo and labeled invading tumor cells up to 3.5 mm away from the tumor margin. Molecular targeting peptides can be combined to form dual targeting probes. Targeting peptide AS1411 labeled with Cy3 showed a significantly higher uptake in glioma cells when combined with the BBB targeting peptide TGN [61]. Dual targeting of integrin and matrix metallo-proteinase (MMP-2) showed in vivo a TNR of 7.8 and in vitro an improved uptake compared to integrin and MMP targeting alone [18].
A metabolic targeting agent is the alkylphosphocholine analog (CLR1502). This was compared with 5-ALA in a mouse model, showing a significant higher TNR (9.28 vs. 4.81) [104].
Two passive targeting fluorophores were identified [4, 77]. A mouse study showed that the CH1055 molecule has a maximal TNR of 5.50 ± 0.36. The authors speculate that, in the future, this molecule could also be conjugated to anti-EGFR affibodies to increase the TNR [4]. Furthermore, motexafin gadolinium was shown to be a feasible marker for gliomas in a rat glioma model both with optical imaging and on T1 MRI [77].
Nanoparticles
Twelve papers evaluated nanoparticles in a preclinical setting with molecular (eight studies), metabolic (two), and passive (two) targeting mechanisms [10, 19, 46–48, 51, 68, 73, 83, 90, 108, 121]. Quantum dots (QDs) are nanoparticles constructed from semiconducting nanocrystals and can function as fluorescent ‘dye’ due to their optical properties. Quantum dots have a tunable emission wavelength based on the diameter and stable fluorescence activity. They can be used as imaging or tumor-targeting agents, and specific peptides coated on the surface can modify their function [76].
QDs coated with RGD peptides (QD-RGDs) specifically target integrin molecules expressed by GBM cells. In vivo, fluorescence imaging of QD-RDGs showed a TNR of 4.42. This was significantly higher than for QDs without RDGs coated on their shell [10]. The peptide F3, which targets the tumor cell surface receptor nucleolin, enhances uptake of the fluorescent polyacrylamide nanoparticles in glioma cells by a factor of 3.1 compared to nanoparticles without F3 [73]. One study investigated FGS in mice with selective porphyrin-based nanostructure mimicking nature lipoproteins (PLP). In vivo confocal microscopy showed tumor delineation at the cellular level. FGS resulted in minimal residual tumor cells in the resection cavity [19]. Dual targeting upconversion nanoparticles (nanoparticles that are capable of absorbing two or more low-energy photons and emitting one high-energy photon) were labeled with angiopeptide-2 and PEG (ANG/PEG-UCNPs) to cross the BBB and target GBM cells in mice. Due to their bimodal imaging properties, ANG/PEG-UCNPs can be used for MRI diagnosis and fluorescence imaging for surgery [68]. Magnetic ironoxide nanoparticles use these bimodal imaging properties as well. An iron oxide nanoparticle labeled with polyethylene glycol-block-polycaprolactone (PEG-b-PCL) and the glioma-targeting ligand lactoferrin (Lf), showed a TNR of 3.8 in a mouse model [121]. Molecular targeting with lactoferrin is also performed with a polymer-based nanoparticle [47].
Cross-linked iron oxide (CLIO) labeled with Cy5.5 is a metabolic targeting nanoparticle that is internalized and accumulated in tumor cells within a maximum of 24 h after injection [51, 108]. Uptake of CLIO-Cy5.5 was also seen in microglia and macrophages at the tumor boarder, resulting in an overestimation of fluorescent enhancement beyond the tumor border between 2 and 24 μm in mice and rat models. No uptake was seen in neurons [108].
Evans Blue (EB) is a passive fluorescent agent that falsely stains healthy tissue due to diffusion. EB capsuled in a liposomal nanoparticle (nano-EB), however, showed a sensitivity and specificity in discriminating tumor from brain tissue of 89 and 100%, respectively [83]. Nano-EB did not stain healthy brain tissue, but underestimated the true margin on the order of tens to hundreds of micrometers, as reported in a rat study. High-dosed QDs coated with polyethylene glycol (PEG) are phagocytized by tumor-induced inflammatory cells (macrophages and microglia) in the tumor border, but not by tumor or brain cells. A study showed that by using QD-PEGs, the tumor margin and satellite lesions could be visualized in vivo in rats [46].
Anti-EGFR or anti-EGF
Four preclinical studies evaluated anti-EGFR antibodies or EGF labeled with a fluorescent compound to discriminate tumor cells from adjacent brain tissue [21, 48, 90, 91]. Epidermal growth factor receptor (EGFR) is a cell-surface receptor overexpressed in many cancer types, including glioma. Gliomas express the wild-type or mutated forms of EGFR, including the GBM specific EGFRvIII. In a mouse model, glioma cells were injected in the brain and 2 weeks later nanoparticles (gold nanorods, GNR) labeled with anti-EGFR antibodies were injected intravenously. Post-mortem imaging of their brain showed a strong absorption in malignant tissue areas [90]. In a combined human and animal ex vivo study, labeling quantum dots (QDs) with EGF and anti-EGFR antibodies visualized individual tumor cells with confocal imaging reaching a TNR as high as 1000, even for LGGs. QDs bound to a combination of EGF and several EGFR antibodies were able to target mutated forms of EGFR as the GBM specific EGFRvIII [48]. In vivo imaging with MRI- fluorescence molecular tomography (MRI-FMT) of mice injected with IRDye 8000CW labeled EGF, showed a 100% sensitivity and specificity in distinguishing mice with EGFR (+) tumor cell lines from EGRF (−) tumor cell lines or control mice. Histological accuracy in distinguishing brain and tumor tissue was not calculated, however [21]. In a recent mouse study, the smaller anti-EGFR affibody protein (±7kDA) had a significantly higher concentration in the tumor periphery than the full antibody (±150 kDa) [91]. Molecular targeting of EGFR is a promising development in FGS; however, it is dependent on the expression of EGFR in tumor cells.
Discussion
Various fluorescent agents have been studied for use in glioma surgery, of which 5-ALA, ICG, fluorescein, hypericin, AFL-HSA, and endogenous spectroscopy have been tested clinically (Table 3).
The three RCTs demonstrated that the use of 5-ALA-based FGS results in improved extent of resection in FGS for glioma [99], and improved PFS [26, 101]. Observational cohort studies suggest that the use of fluorescein increases the rate of GTR as well [11, 52, 92], and that it has a positive effect on PFS [11]. To date, the evidence for effectiveness of clinically tested fluorescent agents other than 5-ALA has been based on only observational cohort studies and case series. Selection bias is a major factor influencing the results in these studies. A direct comparison between 5-ALA and other fluorescent agents is therefore not possible and would require additional, specifically designed studies, however.
Methodological heterogeneity reduces comparability of the studies. Several of the clinical 5-ALA studies specifically included gliomas in eloquent areas, which could have resulted in a lower GTR rate, PFS, and overall survival compared to gliomas in surgically favorable locations [22, 31, 89]. In future studies, parameters such as tumor localization should be included so that relevant corrections can be made. 5-ALA but also fluorescein and ICG have been evaluated in combination with additional intraoperative tools to increase the visualization of the tumor margin and the extent of resection, thereby reducing the comparability of different studies. Different timing and dose of the fluorescent agent add to the differences between the studies as well. Fluorescein, for example, was administered intravenously at the time of anesthesia induction [23] or opening of the dura mater [52] with dosage regimens ranging from 3 mg/kg [23] to 20 mg/kg [52]. Also, it is essential that a more standard definition of GTR is used. In most of the selected studies, GTR was defined as absence of contrast enhancement on post-operative MRI [27]. Other definitions included a reduction of more than 98% of the tumor volume based on volumetric measures [31], or less than 0.175 cm3 contrast enhancement on the post-operative MRI [26]. Instead of GTR, some authors report volumetric differences between pre- and post-operative MRI [15, 25]. Furthermore, the timing of the post-operative MRI varied between the studies from less than 24 h [52], less than 72 h [99], less than 1 week [11] to up to even 1 month [92] after surgery. Often, no details were provided by whom the post-operative MRIs were evaluated and if they were blinded to the procedure performed [11]. This variety in timing, reading of the images, and blinding affect the quality of assessment and comparability of the reported GTR rates among all studies.
Reported sensitivities and specificities of the various agents to distinguish brain from tumor tissue vary greatly between the included studies. Observational studies suggest that all clinically tested exogenous agents had a lower histological accuracy in LGGs compared to HGG [28, 33, 35, 86, 109, 110, 113]. In contrast, endogenous fluorophores showed a higher histological accuracy in LGGs compared to HGGs [9, 118]. However, this outcome measure is very susceptible to bias given the lack of uniform agreement on what samples should be studied. The results are very dependent on the number, timing, and location of biopsy samples taken during surgery. These details are often lacking or described in a non-reproducible and non-comparable fashion.
Pre-clinically, many fluorescent agents with different (more targeted) mechanisms of action are being developed and tested for FGS for glioma (Table 4). Agents targeting EGFR (vIII) show promising histological accuracy results [48]. It should be noted, however, that the included studies were extremely heterogeneous in study design. Furthermore, pre-clinically tested agents were not used as guidance during surgery in patients but mostly assessed on their histological accuracy in ex vivo and in vitro models. A comparison between pre-clinically and clinically tested agents based on these reports is therefore not possible.
Previously, three excellent meta-analyses evaluated the effect FGS on GTR rate and survival [27, 103, 120]. All three included HGG patients only, however, two of which were limited to 5-ALA alone [27, 120] and one to 5-ALA, fluorescein, and hypericin [103]. One paper comprehensively reviewed only the clinically tested exogenous agents though [57]. A more recent systematic review focused on pre-clinically tested molecular targeting agents for visualizing GBM tissue [17]. This systematic review does not include all pre-clinically tested agents, however. To our knowledge, this is the first paper that systematically reviews all existing literature on all pre-clinically and clinically tested contrast agents for FGS in low- and high-grade gliomas.
Challenges in evaluating fluorescent agents and future research
The evaluation of fluorescent agents has many challenges. For the purpose of this review, we chose the rate of GTR, PFS, overall survival, and histological accuracy (sensitivity, specificity, TNR) as outcome measures, because these are the most frequently reported outcome measures among these studies. This does not necessarily mean that these are the most appropriate measures to evaluate fluorescent agents. As indicated by Stummer et al. in 2011, the 5-ALA study was designed for testing the efficacy and safety of 5-ALA as a surgical tool and a diagnostic drug for glioma surgery. In the process of developing the 5-ALA study, the European Medical Evaluations Agency advised to test the agent in a prospective, randomized setting according to the same standards as those for cytotoxic drugs [101]. The study of Schebesch et al. in 2013 demonstrated that FGS can also be evaluated by classifying them as ‘helpful’ or ‘not helpful’ by the operating neurosurgeon [86]. Even though this might be less objective than the outcome measures included in this review, subjective outcomes like this are nevertheless very helpful for the practicing neurosurgeon.
Furthermore, GTR rate and PFS are radiological outcome measures used as indicators for clinical outcome. Overall survival, neurological symptoms, need for re-resection or adjuvant therapy, and quality-of-life assessments would be examples of other, perhaps more direct clinical outcomes that could be used, although these may be more difficult to assess and quantify. If GTR and PFS are to be used as indicators for clinical outcome, what would be the cut-off value to pursue? Residual tumor tissue on the post-operative MRI is shown to result in a decrease in overall survival, but the absolute differences in median post-operative tumor volume were very small (0 cm3 in the 5-ALA group vs. 0.5 cm3 in the control group) in the two RCTs of Stummer [99, 101]. Defining to what extent tumor resection is clinically relevant helps not only in standardizing the definition of GTR for comparison between studies but also aids in balancing maximal cytoreduction and preservation of functional outcome.
Well-designed trials to evaluate the safety and effectiveness of different fluorescent agents before introduction in the clinic are essential. We recognize, however, that RCTs for this purpose offer specific challenges, and applaud the efforts by Stummer et al. in evaluating a diagnostic and surgical tool according to therapeutic standards. Other challenges to be overcome include the impossibility of a double-blind study design in this context, as the surgeon cannot be blinded for the use of fluorescent agents, the potential learning curve in the clinical application of these products, and inter- and intra-surgeon variability. Despite these challenges, the results of both pre-clinical and clinical studies on fluorescent agents for use in glioma surgery provide a growing body of evidence of both effectiveness and safety that will likely continue to develop as these products are transitioned more frequently into clinical practice.
Conclusions
In FGS for glioma, fluorescent agents should be easy to apply, safe to use, and tumor-specific. The fluorescent signal should be strong and easy to detect. Currently, 5-ALA is the only agent that has been tested in a multi-center RCT and has been approved for clinical use in certain parts of the world. Other clinically tested exogenous agents for FGS for glioma include fluorescein, ICG, AFL-HSA, and hypericin. Despite their contributions to GTR, due to their non-specific mechanism of action, preclinical research has shifted away from these products and towards molecular targeting (e.g., anti-EGFR). As histological accuracy increases with the improvement of fluorescent agents, there will be emerging interest in visualization at the cellular level with imaging systems like confocal microscopy. Currently, direct comparisons between the various agents are not possible and would require additional studies. Future studies could make such comparisons possible by using a more standardized, uniform design, with improved definitions of GTR and a broader set of outcome measures.
Abbreviations
- 5-ALA:
-
5-aminolevulinic acid
- BBB:
-
Blood–brain barrier
- EGFR:
-
Epidermal growth factor receptor
- FGS:
-
Fluorescence-guided surgery
- GTR:
-
Gross total resection
- HGG:
-
High-grade glioma
- ICG:
-
Indocyanine green
- LGG:
-
Low-grade glioma
- MRI:
-
Magnetic resonance imaging
- NIR:
-
Near-infrared
- PFS:
-
Progression-free survival
- PpIX:
-
Protoporphyrin
- QD:
-
Quantum dot
- TNR:
-
Tumor-to-normal ratio
References
Acerbi F, Broggi M, Eoli M, Anghileri E, Cavallo C, Boffano C, Cordella R, Cuppini L, Pollo B, Schiariti M, Visintini S, Orsi C, La Corte E, Broggi G, Ferroli P (2014) Is fluorescein-guided technique able to help in resection of high-grade gliomas? Neurosurg Focus 36, E5
Agnes RS, Broome AM, Wang J, Verma A, Lavik K, Basilion JP (2012) An optical probe for noninvasive molecular imaging of orthotopic brain tumors overexpressing epidermal growth factor receptor. Mol Cancer Ther 11:2202–2211. doi:10.1158/1535-7163.MCT-12-0211
Aldave G, Tejada S, Pay E, Marigil M, Bejarano B, Idoate MA, Diez-Valle R (2013) Prognostic value of residual fluorescent tissue in glioblastoma patients after gross total resection in 5-aminolevulinic acid-guided surgery. Neurosurgery 72:915–920. doi:10.1227/NEU.0b013e31828c3974, discussion 920–911
Antaris AL, Chen H, Cheng K, Sun Y, Hong G, Qu C, Diao S, Deng Z, Hu X, Zhang B, Zhang X, Yaghi OK, Alamparambil ZR, Hong X, Cheng Z, Dai H (2016) A small-molecule dye for NIR-II imaging. Nat Mater 15:235–242. doi:10.1038/nmat4476
Barbagallo GMV, Palmucci S, Visocchi M, Paratore S, Attinà G, Sortino G, Albanese V, Certo F (2016) Portable intraoperative computed tomography scan in image-guided surgery for brain high-grade gliomas: analysis of technical feasibility and impact on extent of tumor resection. Oper Neurosurg 12:19–30
Belloch JP, Rovira V, Llácer JL, Riesgo PA, Cremades A (2014) Fluorescence-guided surgery in high-grade gliomas using an exoscope system. Acta Neurochir 156:653–660
Burden-Gulley SM, Zhou Z, Craig SEL, Lu ZR, Brady-Kalnay SM (2013) Molecular magnetic resonance imaging of tumors with a PTPμ targeted contrast agent. Transl Oncol 6:329–337
Butte PV, Mamelak A, Parrish-Novak J, Drazin D, Shweikeh F, Gangalum PR, Chesnokova A, Ljubimova JY, Black K (2014) Near-infrared imaging of brain tumors using the tumor paint BLZ-100 to achieve near-complete resection of brain tumors. Neurosurg Focus 36, E1. doi:10.3171/2013.11.FOCUS13497
Butte PV, Mamelak AN, Nuno M, Bannykh SI, Black KL, Marcu L (2011) Fluorescence lifetime spectroscopy for guided therapy of brain tumors. NeuroImage 54:S125–S135
Cai W, Shin DW, Chen K, Gheysens O, Cao Q, Wang SX, Gambhir SS, Chen X (2006) Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett 6:669–676
Chen B, Wang H, Ge P, Zhao J, Li W, Gu H, Wang G, Luo Y, Chen D (2012) Gross total resection of glioma with the intraoperative fluorescence-guidance of fluorescein sodium. Int J Med Sci 9:708–714
Chung IW, Eljamel S (2013) Risk factors for developing oral 5-aminolevulinic acid-induced side effects in patients undergoing fluorescence-guided resection. Photodiagn Photodyn Ther 10:362–367. doi:10.1016/j.pdpdt.2013.03.007
Coburger J, Engelke J, Scheuerle A, Thal DR, Hlavac M, Wirtz CR, König R (2014) Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA-enhanced intraoperative MRI at the border of contrast-enhancing lesions: a prospective study based on histopathological assessment. Neurosurg Focus 36, E3
Coburger J, Hagel V, Wirtz CR, König R (2015) Surgery for glioblastoma: impact of the combined use of 5-aminolevulinic acid and intraoperative MRI on extent of resection and survival. PLoS ONE 10
Cordova JS, Gurbani SS, Holder CA, Olson JJ, Schreibmann E, Shi R, Guo Y, Shu HG, Shim H, Hadjipanayis CG (2015) Semi-automated volumetric and morphological assessment of glioblastoma resection with fluorescence-guided surgery. Mol Imaging Biol MIB Off Pub Acad Mole Imaging. doi:10.1007/s11307-015-0900-2
Cortnum S, Laursen RJ (2012) Fluorescence-guided resection of gliomas. Dan Med J 59:A4460
Craig SE, Wright J, Sloan AE, Brady-Kalnay SM (2016) Fluorescent-guided surgical resection of glioma with targeted molecular imaging agents: a literature review. World Neurol 90:154–163. doi:10.1016/j.wneu.2016.02.060
Crisp JL, Savariar EN, Glasgow HL, Ellies LG, Whitney MA, Tsien RY (2014) Dual targeting of integrin αvβ3 and matrix metalloproteinase-2 for optical imaging of tumors and chemotherapeutic delivery. Mol Cancer Ther 13:1514–1525
Cui L, Lin Q, Jin CS, Jiang W, Huang H, Ding L, Muhanna N, Irish JC, Wang F, Chen J, Zheng G (2015) A PEGylation-free biomimetic porphyrin nanoplatform for personalized cancer theranostics. ACS Nano 9:4484–4495
Cutter JL, Cohen NT, Wang J, Sloan AE, Cohen AR, Panneerselvam A, Schluchter M, Blum G, Bogyo M, Basilion JP (2012) Topical application of activity-based probes for visualization of brain tumor tissue. PLoS One 7, e33060. doi:10.1371/journal.pone.0033060
Davis SC, Samkoe KS, O’Hara JA, Gibbs-Strauss SL, Payne HL, Hoopes PJ, Paulsen KD, Pogue BW (2010) MRI-coupled fluorescence tomography quantifies EGFR activity in brain tumors. Acad Radiol 17:271–276
Della Puppa A, De Pellegrin S, D’Avella E, Gioffrè G, Rossetto M, Gerardi A, Lombardi G, Manara R, Munari M, Saladini M, Scienza R (2013) 5-aminolevulinic acid (5-ALA) fluorescence-guided surgery of high-grade gliomas in eloquent areas assisted by functional mapping. Our experience and review of the literature. Acta Neurochir 155:965–972
Diaz RJ, Dios RR, Hattab EM, Burrell K, Rakopoulos P, Sabha N, Hawkins C, Zadeh G, Rutka JT, Cohen-Gadol AA (2015) Study of the biodistribution of fluorescein in glioma-infiltrated mouse brain and histopathological correlation of intraoperative findings in high-grade gliomas resected under fluorescein fluorescence guidance. J Neurosurg 122:1360–1369
Diez Valle R, Slof J, Galvan J, Arza C, Romariz C, Vidal C, Dr Valle RD, PT Rodriguez I, Martinez GV, Dr Cabezudo JM, Dr Garcia LMB, Dr Sanchez JJG, Dr Rodriguez EF, Dr Sanchez MAA, Dr Granados GO, Dr Delgado AT, Dr Bertran GC, Dr Martin JJA, Dr Ahicart GP, Dr Diaz AP, Dr Asuncion CB, Dr Acin RP, de Pedro MA, Dr Urzaiz LL, Dr Barcia JA, Dr Brin JR, Dr Campa-Santamarina JMT, Dr Sanchez AM, Dr Pena JM (2014) Observational, retrospective study of the effectiveness of 5-aminolevulinic acid in malignant glioma surgery in Spain (The VISIONA study). Estudio observacional retrospectivo sobre la efectividad del acido 5-aminolevulinico en la cirugia de los gliomas malignos en Espana (Estudio VISIONA) 29:131–138
Díez Valle R, Tejada Solis S, Idoate Gastearena MA, García De Eulate R, Domínguez Echávarri P, Aristu Mendiroz J (2011) Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: volumetric analysis of extent of resection in single-center experience. J Neuro-Oncol 102:105–113
Eljamel MS, Goodman C, Moseley H (2008) ALA and photofrin® fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: a single centre phase III randomised controlled trial. Lasers Med Sci 23:361–367
Eljamel S (2015) 5-ALA fluorescence image guided resection of glioblastoma multiforme: a meta-analysis of the literature. Int J Mol Sci 16:10443–10456
Ewelt C, Floeth FW, Felsberg J, Steiger HJ, Sabel M, Langen KJ, Stoffels G, Stummer W (2011) Finding the anaplastic focus in diffuse gliomas: the value of Gd-DTPA enhanced MRI, FET-PET, and intraoperative, ALA-derived tissue fluorescence. Clin Neurol Neurosurg 113:541–547
Eyupoglu IY, Hore N, Fan Z, Buslei R, Merkel A, Buchfelder M, Savaskan NE (2015) Intraoperative vascular DIVA surgery reveals angiogenic hotspots in tumor zones of malignant gliomas. Sci Report 5:7958. doi:10.1038/srep07958
Eyüpoglu IY, Hore N, Savaskan NE, Grummich P, Roessler K, Buchfelder M, Ganslandt O (2012) Improving the extent of malignant glioma resection by dual intraoperative visualization approach. PLoS ONE. doi:10.1371/journal.pone.0044885
Feigl GC, Ritz R, Moraes M, Klein J, Ramina K, Gharabaghi A, Krischek B, Danz S, Bornemann A, Liebsch M, Tatagiba MS (2010) Resection of malignant brain tumors in eloquent cortical areas: a new multimodal approach combining 5-aminolevulinic acid and intraoperative monitoring. J Neurosurg 113:352–357
Fenton KE, Martirosyan NL, Abdelwahab MG, Coons SW, Preul MC, Scheck AC (2014) In vivo visualization of GL261-luc2 mouse glioma cells by use of Alexa fluor-labeled TRP-2 antibodies. Neurosurg Focus 36, E12
Floeth FW, Sabel M, Ewelt C, Stummer W, Felsberg J, Reifenberger G, Steiger HJ, Stoffels G, Coenen HH, Langen KJ (2011) Comparison of 18F-FET PET and 5-ALA fluorescence in cerebral gliomas. Eur J Nucl Med Mol Imaging 38:731–741
Gessler F, Forster MT, Duetzmann S, Mittelbronn M, Hattingen E, Franz K, Seifert V, Senft C (2015) Combination of intraoperative magnetic resonance imaging and intraoperative fluorescence to enhance the resection of contrast enhancing gliomas. Neurosurgery 77:16–22
Haglund MM, Berger MS, Hochman DW (1996) Enhanced optical imaging of human gliomas and tumor margins. Neurosurgery 38:308–317
Haglund MM, Hochman DW, Spence AM, Berger MS (1994) Enhanced optical imaging of rat gliomas and tumor margins. Neurosurgery 35:930–940, discussion 940–931
Haj-Hosseini N, Richter JCO, Hallbeck M, Wardell K (2015) Low dose 5-aminolevulinic acid: implications in spectroscopic measurements during brain tumor surgery. Photodiagn Photodyn Ther 12:209–2014
Hamamcioglu MK, Akcakaya MO, Goker B, Kasimcan MO, Kiris T (2016) The use of the YELLOW 560nm surgical microscope filter for sodium fluorescein-guided resection of brain tumors: our preliminary results in a series of 28 patients. Clin Neurol Neurosurg 143:39–45. doi:10.1016/j.clineuro.2016.02.006
Hansen DA, Spence AM, Carski T, Berger MS (1993) Indocyanine green (ICG) staining and demarcation of tumor margins in a rat glioma model. Surg Neurol 40:451–456
Hauser SB, Kockro RA, Actor B, Sarnthein J, Bernays RL (2016) Combining 5-aminolevulinic acid fluorescence and intraoperative magnetic resonance imaging in glioblastoma surgery: a histology-based evaluation. Neurosurgery 78:475–483
Hefti M, Von Campe G, Moschopulos M, Siegner A, Looser H, Landolt H (2008) 5-Aminolaevulinic acid-induced protoporphyrin IX fluorescence in high-grade glioma surgery. Swiss Med Wkly 138:180–185
Hickmann AK, Nadji-Ohl M, Hopf NJ (2015) Feasibility of fluorescence-guided resection of recurrent gliomas using five-aminolevulinic acid: retrospective analysis of surgical and neurological outcome in 58 patients. J Neuro-Oncol 122:151–160
Huang R, Vider J, Kovar JL, Olive DM, Mellinghoff IK, Mayer-Kuckuk P, Kircher MF, Blasberg RG (2012) Integrin αvβ3-targeted IRDye 800CW near-infrared imaging of glioblastoma. Clin Cancer Res 18:5731–5740
Idoate MA, Diez Valle R, Echeveste J, Tejada S (2011) Pathological characterization of the glioblastoma border as shown during surgery using 5-aminolevulinic acid-induced fluorescence. Neuropathology 31:575–582. doi:10.1111/j.1440-1789.2011.01202.x
Irwin CP, Portorreal Y, Brand C, Zhang Y, Desai P, Salinas B, Weber WA, Reiner T (2014) PARPi-FL—a fluorescent PARP1 inhibitor for glioblastoma imaging. Neoplasia 16:432–440. doi:10.1016/j.neo.2014.05.005
Jackson H, Muhammad O, Daneshvar H, Nelms J, Popescu A, Vogelbaum MA, Bruchez M, Toms SA (2007) Quantum dots are phagocytized by macrophages and colocalize with experimental gliomas. Neurosurgery 60:524–529
Jiang L, Zhou Q, Mu K, Xie H, Zhu Y, Zhu W, Zhao Y, Xu H, Yang X (2013) PH/temperature sensitive magnetic nanogels conjugated with Cy5.5-labled lactoferrin for MR and fluorescence imaging of glioma in rats. Biomaterials 34:7418–7428
Kantelhardt SR, Caarls W, de Vries AHB, Hagen GM, Jovin TM, Schulz-Schaeffer W, Rohde V, Giese A, Arndt-Jovin DJ (2010) Specific visualization of glioma cells in living low-grade tumor tissue. PLoS ONE 5
Kantelhardt SR, Kalasauskas D, Konig K, Kim E, Weinigel M, Uchugonova A, Giese A (2016) In vivo multiphoton tomography and fluorescence lifetime imaging of human brain tumor tissue. J Neuro-Oncol. doi:10.1007/s11060-016-2062-8
Kantelhardt SR, Leppert J, Kantelhardt JW, Reusche E, Huttmann G, Giese A (2009) Multi-photon excitation fluorescence microscopy of brain-tumour tissue and analysis of cell density. Acta Neurochir 151:253–262. doi:10.1007/s00701-009-0188-6, discussion 262
Kircher MF, Mahmood U, King RS, Weissleder R, Josephson L (2003) A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res 63:8122–8125
Koc K, Anik I, Cabuk B, Ceylan S (2008) Fluorescein sodium-guided surgery in glioblastoma multiforme: a prospective evaluation. Br J Neurosurg 22:99–103
Kremer P, Fardanesh M, Ding R, Pritsch M, Zoubaa S, Frei E (2009) Intraoperative fluorescence staining of malignant brain tumors using 5-aminofluorescein-labeled albumin. Neurosurgery 64:ons53-60; discussion ons60-51. doi:10.1227/01.neu.0000335787.17029.67
Kuroiwa T, Kajimoto Y, Ohta T (1998) Development of a fluorescein operative microscope for use during malignant glioma surgery a technical note and preliminary report. Surg Neurol 50:41–49
Lanzardo S, Conti L, Brioschi C, Bartolomeo MP, Arosio D, Belvisi L, Manzoni L, Maiocchi A, Maisano F, Forni G (2011) A new optical imaging probe targeting alphaVbeta3 integrin in glioblastoma xenografts. Contrast Media Mol Imaging 6:449–458. doi:10.1002/cmmi.444
Leppert J, Krajewski J, Kantelhardt SR, Schlaffer S, Petkus N, Reusche E, Hüttmann G, Giese A (2006) Multiphoton excitation of autofluorescence for microscopy of glioma tissue. Neurosurgery 58:759–767
Li Y, Rey-Dios R, Roberts DW, Valdes PA, Cohen-Gadol AA (2014) Intraoperative fluorescence-guided resection of high-grade gliomas: a comparison of the present techniques and evolution of future strategies. World Neurol 82:175–185. doi:10.1016/j.wneu.2013.06.014
Lin WC, Toms SA, Johnson M, Jansen ED, Mahadevan-Jansen A (2001) In vivo brain tumor demarcation using optical spectroscopy. Photochem Photobiol 73:396–402
Liu JTC, Meza D, Sanai N (2014) Trends in fluorescence image-guided surgery for gliomas. Neurosurgery 75:61–71
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. doi:10.1007/s00401-016-1545-1
Ma H, Gao Z, Yu P, Shen S, Liu Y, Xu B (2014) A dual functional fluorescent probe for glioma imaging mediated by blood–brain barrier penetration and glioma cell targeting. Biochem Biophys Res Commun 449:44–48
Marcu L, Jo JA, Butte PV, Yong WH, Pikul BK, Black KL, Thompson RC (2004) Fluorescence lifetime spectroscopy of glioblastoma multiforme. Photochem Photobiol 80:98–103. doi:10.1562/2003-12-09-RA-023.1
Martirosyan NL, Cavalcanti DD, Eschbacher JM, Delaney PM, Scheck AEC, Abdelwahab MG, Nakaji P, Spetzler RF, Preul MC (2011) Use of in vivo near-infrared laser confocal endomicroscopy with indocyanine green to detect the boundary of infiltrative tumor: laboratory investigation. J Neurosurg 115:1131–1138
Martirosyan NL, Eschbacher JM, Kalani MY, Turner JD, Belykh E, Spetzler RF, Nakaji P, Preul MC (2016) Prospective evaluation of the utility of intraoperative confocal laser endomicroscopy in patients with brain neoplasms using fluorescein sodium: experience with 74 cases. Neurosurg Focus 40, E11. doi:10.3171/2016.1.focus15559
Moore GE, Peyton WT (1948) The clinical use of fluorescein in neurosurgery; the localization of brain tumors. J Neurosurg 5:392–398. doi:10.3171/jns.1948.5.4.0392
Nabavi A, Thurm H, Zountsas B, Pietsch T, Lanfermann H, Pichlmeier U, Mehdorn M (2009) Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: a phase II study. Neurosurgery 65:1070–1076
Nguyen QT, Tsien RY (2013) Fluorescence-guided surgery with live molecular navigation—a new cutting edge. Nat Rev Cancer 13:653–662. doi:10.1038/nrc3566
Ni D, Zhang J, Bu W, Xing H, Han F, Xiao Q, Yao Z, Chen F, He Q, Liu J, Zhang S, Fan W, Zhou L, Peng W, Shi J (2014) Dual-targeting upconversion nanoprobes across the blood–brain barrier for magnetic resonance/fluorescence imaging of intracranial glioblastoma. ACS Nano 8:1231–1242
Noell S, Feigl GC, Naros G, Barking S, Tatagiba M, Ritz R (2015) Experiences in surgery of primary malignant brain tumours in the primary sensori-motor cortex practical recommendations and results of a single institution. Clin Neurol Neurosurg 136:41–50
Noell S, Mayer D, Strauss WS, Tatagiba MS, Ritz R (2011) Selective enrichment of hypericin in malignant glioma: pioneering in vivo results. Int J Oncol 38:1343–1348. doi:10.3892/ijo.2011.968
Okuda T, Yoshioka H, Kato A (2012) Fluorescence-guided surgery for glioblastoma multiforme using high-dose fluorescein sodium with excitation and barrier filters. J Clin Neurosci 19:1719–1722
Olsen TW, Lim JI, Capone A Jr, Myles RA, Gilman JP (1996) Anaphylactic shock following indocyanine green angiography. Arch Ophthalmol 114:97
Orringer DA, Koo YEL, Chen T, Kim G, Hah HJ, Xu H, Wang S, Keep R, Philbert MA, Kopelman R, Sagher O (2009) In vitro characterization of a targeted, dye-loaded nanodevice for intraoperative tumor delineation. Neurosurgery 64:965–971
Panciani PP, Fontanella M, Schatlo B, Garbossa D, Agnoletti A, Ducati A, Lanotte M (2012) Fluorescence and image guided resection in high-grade glioma. Clin Neurol Neurosurg 114:37–41
Piquer J, Llácer JL, Rovira V, Riesgo P, Rodriguez R, Cremades A (2014) Fluorescence-guided surgery and biopsy in gliomas with an exoscope system. Biomed Res Int. doi:10.1155/2014/207974
Pogue BW, Gibbs-Strauss S, Valdes PA, Samkoe K, Roberts DW, Paulsen KD (2010) Review of neurosurgical fluorescence imaging methodologies. IEEE J Sel Top Quantum Electron 16:493–505. doi:10.1109/JSTQE.2009.2034541
Qiu L, Zhang F, Shi Y, Bai Z, Wang J, Li Y, Lee D, Ingraham C, Feng X, Yang X (2015) Gliomas: motexafin gadolinium-enhanced molecular MR imaging and optical imaging for potential intraoperative delineation of tumor margins. Radiology. doi:10.1148/radiol.2015150895
Quick-Weller J, Lescher S, Forster MT, Konczalla J, Seifert V, Senft C (2016) Combination of 5-ALA and iMRI in re-resection of recurrent glioblastoma. Br J Neurosurg. doi:10.3109/02688697.2015.1119242
Riemann I, Le Harzic R, Mpoukouvalas K, Heimann A, Kempski O, Charalampaki P (2012) Sub-cellular tumor identification and markerless differentiation in the rat brain in vivo by multiphoton microscopy. Lasers Surg Med 44:719–725
Ritz R, Daniels R, Noell S, Feigl GC, Schmidt V, Bornemann A, Ramina K, Mayer D, Dietz K, Strauss WSL, Tatagiba M (2012) Hypericin for visualization of high-grade gliomas: first clinical experience. Eur J Surg Oncol 38:352–360
Roberts DW, Valdes PA, Harris BT, Fontaine KM, Hartov A, Fan X, Ji S, Lollis SS, Pogue BW, Leblond F, Tosteson TD, Wilson BC, Paulsen KD (2011) Coregistered fluorescence-enhanced tumor resection of malignant glioma: relationships between delta-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical Article J Neurosurg 114:595–603. doi:10.3171/2010.2.jns091322
Roder C, Bisdas S, Ebner FH, Honegger J, Naegele T, Ernemann U, Tatagiba M (2014) Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: high-field iMRI versus conventional and 5-ALA-assisted surgery. Eur J Surg Oncol 40:297–304
Roller BT, Munson JM, Brahma B, Santangelo PJ, Pai SB, Bellamkonda RV (2015) Evans blue nanocarriers visually demarcate margins of invasive gliomas. Drug Deliv Transl Res 5:116–124
Sanai N, Snyder LA, Honea NJ, Coons SW, Eschbacher JM, Smith KA, Spetzler RF (2011) Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low-grade gliomas: clinical article. J Neurosurg 115:740–748
Schatlo B, Fandino J, Smoll NR, Wetzel O, Remonda L, Marbacher S, Perrig W, Landolt H, Fathi AR (2015) Outcomes after combined use of intraoperative MRI and 5-aminolevulinic acid in high-grade glioma surgery. Neuro-Oncology 17:1560–1567
Schebesch KM, Proescholdt M, Höhne J, Hohenberger C, Hansen E, Riemenschneider MJ, Ullrich W, Doenitz C, Schlaier J, Lange M, Brawanski A (2013) Sodium fluorescein-guided resection under the YELLOW 560 nm surgical microscope filter in malignant brain tumor surgery—a feasibility study. Acta Neurochir 155:693–699
Schucht P, Beck J, Abu-Isa J, Andereggen L, Murek M, Seidel K, Stieglitz L, Raabe A (2013) Gross total resection rates in glioblastoma surgery: combining 5-ALA intraoperative fluorescence imaging and brain mapping. J Neurosurg 119:A539–A540
Schucht P, Beck J, Seidel K, Raabe A (2015) Extending resection and preserving function: modern concepts of glioma surgery. Swiss Med Wkly 145:w14082. doi:10.4414/smw.2015.14082
Schucht P, Seidel K, Beck J, Murek M, Jilch A, Wiest R, Fung C, Raabe A (2014) Intraoperative monopolar mapping during 5-ALA-guided resections of glioblastomas adjacent to motor eloquent areas: evaluation of resection rates and neurological outcome. Neurosurg Focus 37, E16
Seekell K, Lewis S, Wilson C, Li S, Grant G, Wax A (2013) Feasibility study of brain tumor delineation using immunolabeled gold nanorods. Biomed Opt Express 4:2284–2295
Sexton K, Tichauer K, Samkoe KS, Gunn J, Hoopes PJ, Pogue BW (2013) Fluorescent affibody peptide penetration in glioma margin is superior to full antibody. PLoS ONE. doi:10.1371/journal.pone.0060390
Shinoda J, Yano H, Yoshimura SI, Okumura A, Kaku Y, Iwama T, Sakai N (2003) Fluorescence-guided resection of glioblastoma multiforme by using high-dose fluorescein sodium. Tech Note J Neurosurg 99:597–603
Slof J, Diez Valle R, Galvan J (2015) Cost-effectiveness of 5-aminolevulinic acid-induced fluorescence in malignant glioma surgery. Neurologia 30:163–168. doi:10.1016/j.nrl.2013.11.002
Slotty PJ, Siantidis B, Beez T, Steiger HJ, Sabel M (2013) The impact of improved treatment strategies on overall survival in glioblastoma patients. Acta Neurochir 155:959–963
Soffietti R, Baumert BG, Bello L, Von Deimling A, Duffau H, Frenay M, Grisold W, Grant R, Graus F, Hoang-Xuan K, Klein M, Melin B, Rees J, Siegal T, Smits A, Stupp R, Wick W, European Federation of Neurological S (2010) Guidelines on management of low-grade gliomas: report of an EFNS-EANO task force. Eur J Neurol 17:1124–1133. doi:10.1111/j.1468-1331.2010.03151.x
Spena G, Panciani PP, Fontanella MM (2015) Resection of supratentorial gliomas: the need to merge microsurgical technical cornerstones with modern functional mapping concepts. An Overview. Neurosurg Rev 38:59–70. doi:10.1007/s10143-014-0578-y, discussion 70
Stummer W, Nestler U, Stockhammer F, Krex D, Kern BC, Mehdorn HM, Vince GH, Pichlmeier U (2011) Favorable outcome in the elderly cohort treated by concomitant temozolomide radiochemotherapy in a multicentric phase II safety study of 5-ALA. J Neuro-Oncol 103:361–370
Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ (2000) Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 93:1003–1013
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401
Stummer W, Tonn JC, Goetz C, Ullrich W, Stepp H, Bink A, Pietsch T, Pichlmeier U (2014) 5-aminolevulinic acid-derived tumor fluorescence: the diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery 74:310–319
Stummer W, Tonn JC, Mehdorn HM, Nestler U, Franz K, Goetz C, Bink A, Pichlmeier U (2011) Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study: clinical article. J Neurosurg 114:613–623
Stupp R, Brada M, Van Den Bent MJ, Tonn JC, Pentheroudakis G, Group EGW (2014) High-grade glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 25(3):iii93–101. doi:10.1093/annonc/mdu050
Su X, Huang Q-F, Chen H-L (2014) Fluorescence-guided resection of high-grade gliomas: a systematic review and meta-analysis. Photodiagn Photodyn Ther 11:451–458. doi:10.1016/j.pdpdt.2014.08.001
Swanson KI, Clark PA, Zhang RR, Kandela IK, Farhoud M, Weichert JP, Kuo JS (2015) Fluorescent cancer-selective alkylphosphocholine analogs for intraoperative glioma detection. Neurosurgery 76:115–123
Szmuda T, Sloniewski P, Olijewski W, Springer J, Waszak PM (2015) Colour contrasting between tissues predicts the resection in 5-aminolevulinic acid-guided surgery of malignant gliomas. J Neuro-Oncol 122:575–584. doi:10.1007/s11060-015-1750-0
Teixidor P, Arraez MA, Villalba G, Garcia R, Tardaguila M, Gonzalez JJ, Rimbau J, Vidal X, Montane E (2016) Safety and efficacy of 5-aminolevulinic acid for high-grade glioma in usual clinical practice: a prospective cohort study. PLoS ONE 11
Toms SA, Lin WC, Weil RJ, Johnson MD, Jansen ED, Mahadevan-Jansen A (2005) Intraoperative optical spectroscopy identifies infiltrating glioma margins with high sensitivity. Neurosurgery 57:ONS-382-ONS-390
Tréhin R, Figueiredo JL, Pittet MJ, Weissleder R, Josephson L, Mahmood U (2006) Fluorescent nanoparticle uptake for brain tumor visualization. Neoplasia 8:302–311
Valdes PA, Jacobs V, Harris BT, Wilson BC, Leblond F, Paulsen KD, Roberts DW (2015) Quantitative fluorescence using 5-aminolevulinic acid-induced protoporphyrin IX biomarker as a surgical adjunct in low-grade glioma surgery. J Neurosurg 123:771–780. doi:10.3171/2014.12.JNS14391
Valdés PA, Leblond F, Anthony K, Harris BT, Wilson BC, Fan X, Tosteson TD, Hartov A, Ji S, Erkmen K, Simmons NE, Paulsen KD, Roberts DW (2011) Quantitative fluorescence in intracranial tumor: implications for ALA-induced PpIX as an intraoperative biomarker - clinical article. J Neurosurg 115:11–17
Veiseh M, Gabikian P, Bahrami SB, Veiseh O, Zhang M, Hackman RC, Ravanpay AC, Stroud MR, Kusuma Y, Hansen SJ, Kwok D, Munoz NM, Sze RW, Grady WM, Greenberg NM, Ellenbogen RG, Olson JM (2007) Tumor paint: a chlorotoxin:Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res 67:6882–6888. doi:10.1158/0008-5472.CAN-06-3948
Warram JM, De Boer E, Korb M, Hartman Y, Kovar J, Markert JM, Gillespie GY, Rosenthal EL (2015) Fluorescence-guided resection of experimental malignant glioma using cetuximab-IRDye 800CW. Br J Neurosurg 29:850–858
Widhalm G, Kiesel B, Woehrer A, Traub-Weidinger T, Preusser M, Marosi C, Prayer D, Hainfellner JA, Knosp E, Wolfsberger S (2013) 5-aminolevulinic acid induced fluorescence is a powerful intraoperative marker for precise histopathological grading of gliomas with non-significant contrast-enhancement. PLoS ONE. doi:10.1371/journal.pone.0076988
Wolfbeis OS (2015) An overview of nanoparticles commonly used in fluorescent bioimaging. Chem Soc Rev 44:4743–4768. doi:10.1039/c4cs00392f
Yamada S, Muragaki Y, Maruyama T, Komori T, Okada Y (2015) Role of neurochemical navigation with 5-aminolevulinic acid during intraoperative MRI-guided resection of intracranial malignant gliomas. Clin Neurol Neurosurg 130:134–139
Yan H, Wang J, Yi P, Lei H, Zhan C, Xie C, Feng L, Qian J, Zhu J, Lu W, Li C (2011) Imaging brain tumor by dendrimer-based optical/paramagnetic nanoprobe across the blood–brain barrier. Chem Commun (Camb) 47:8130–8132. doi:10.1039/c1cc12007g
Yang CS, Sung CS, Lee FL, Hsu WM (2007) Management of anaphylactic shock during intravenous fluorescein angiography at an outpatient clinic. J Chin Med Assoc 70:348–349. doi:10.1016/S1726-4901(08)70017-0
Yong WH, Butte PV, Pikul BK, Jo JA, Fang Q, Papaioannou T, Black KL, Marcu L (2006) Distinction of brain tissue, low-grade and high-grade glioma with time-resolved fluorescence spectroscopy. Front Biosci 11:1255–1263
Zehri A, Ramey W, Georges J, Mooney M, Martirosyan N, Preul M, Nakaji P (2014) Neurosurgical confocal endomicroscopy: a review of contrast agents, confocal systems, and future imaging modalities. Surg Neurol Int. doi:10.4103/2152-7806.131638
Zhao S, Wu J, Wang C, Liu H, Dong X, Shi C, Shi C, Liu Y, Teng L, Han D, Chen X, Yang G, Wang L, Shen C, Li H (2013) Intraoperative fluorescence-guided resection of high-grade malignant gliomas using 5-aminolevulinic acid-induced porphyrins: a systematic review and meta-analysis of prospective studies. PLoS ONE. doi:10.1371/journal.pone.0063682
Zhou Q, Mu K, Jiang L, Xie H, Liu W, Li Z, Qi H, Liang S, Xu H, Zhu Y, Zhu W, Yang X (2015) Glioma-targeting micelles for optical/magnetic resonance dual-mode imaging. Int J Nanomedicine 10:1805–1818
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
None.
Human and animal consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Senders, J.T., Muskens, I.S., Schnoor, R. et al. Agents for fluorescence-guided glioma surgery: a systematic review of preclinical and clinical results. Acta Neurochir 159, 151–167 (2017). https://doi.org/10.1007/s00701-016-3028-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-3028-5