Abstract
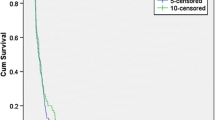
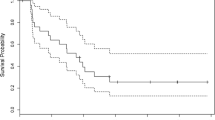
A retrospective evaluation of single agent bevacizumab in adults with recurrent glioblastoma (GBM) with an objective of determining progression free survival (PFS). There is no standard therapy for recurrent GBM after failure of alkylator-based chemotherapy. A total of 50 adults, ages 36–70 years (median 64), with recurrent GBM were treated. All patients had previously been treated with surgery, concurrent radiotherapy and temozolomide, post-radiotherapy temozolomide and in 34 patients, one salvage regimen (PCV: 21, cyclophosphamide: 13). A total of 13 patients underwent repeat surgery. Patients were treated at first or second recurrence with bevacizumab, once every 2 weeks, defined as a single cycle. Neurological evaluation was performed every 2 weeks and neuroradiographic assessment following the initial 2 cycles of bevacizumab and subsequently after every 4 cycles of bevacizumab. A total of 468 cycles of bevacizumab (median 2 cycles; range 1–30) was administered. Bevacizumab-related toxicity included fatigue (16 patients; 4 grade 3), leukopenia (9; 1 grade 3), anemia (5; 0 grade 3), hypertension (7; 1 grade 3), deep vein thrombosis (4; 1 grade 3) and wound dehiscence (2; 1 grade 3). 21 patients (42%) demonstrated a partial radiographic response and 29 (58%) progressive disease following 1–2 cycles of bevacizumab. Time to tumor progression ranged from 0.5 to 15 months (median: 1.0 months). Survival ranged from 2 to 17 months (median: 8.5 months). 6-month and 12-month PFS were 42% and 22% respectively. Single agent bevacizumab demonstrated efficacy and acceptable toxicity in this cohort of adults with recurrent alkylator-refractory GBM.

Similar content being viewed by others
References
The Medical Research Council Brain Tumor Working Party (2001) Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: a Medical Research Council Trial. J Clin Oncol 19(2):509–518
Prados MD, Scott C, Curran WJ et al (1999) Procarbazine, lomustine, and vincristine (PCV) chemotherapy for anaplastic astrocytoma: a retrospective review of Radiation Therapy Oncology Group protocols comparing survival with carmustine or PCV adjuvant chemotherapy. J Clin Oncol 17:3389–3395
Westphal M, Hilt DC, Bortey E et al (2003) A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neurooncology 5(2):79–88
Grossman SA, O’Neill A, Grunnet M, Mehta M et al (2003) Phase III study comparing three cycles of infusional carmustine and cisplatin followed by radiation therapy with radiation therapy and concurrent carmustine in patients with newly diagnosed supratentorial glioblastoma multiforme: Eastern Cooperative Oncology Group Trial 2394. J Clin Oncol 21:1485–1491
Prados MD, Levin V (2000) Biology and treatment of malignant glioma. Semin Oncol 27(Suppl 3):1–10
Gutin PH, Prados MD, Phillips TL et al (1991) External irradiation followed by an interstitial high activity iodine-125 implant “boost” in the initial treatment of malignant gliomas: NCOG Study 6G82-2. Int J Radiat Oncol Biol Phys 21:601
Kornblith PD, Welch WC, Bradley MK (1993) The future of therapy for glioblastoma. Surg Neurol 39:538–543
Loeffler JS, Alexander E, Shea WM et al (1992) Radiosurgery as part of the initial management of patients with malignant gliomas. J Clin Oncol 10(9):1379–1385
Prados MD, Gutin PH, Phillips TL et al (1992) Interstitial brachytherapy for newly diagnosed patients with malignant gliomas: the UCSF experience. Int J Radiat Oncol Biol Phys 24:593
Levin VA, Silver P, Hannigan J et al (1990) Superiority of post-radiotherapy adjuvant chemotherapy with CCNU, procarbazine, and vincristine (PCV) over BCNU for anaplastic gliomas: NCOG 6G61 final report. Int J Radiat Oncol Biol Phys 18:321–324
Stewart LA (2002) Chemotherapy in adult high-grade glioma: a systemic review and meta-analysis of individual patient data from 12 randomized trials. Lancet 359:1011–1018
Fine HA, Dear KB, Loeffler JS et al (1993) Meta-analysis of radiation therapy and without chemotherapy for malignant gliomas in adults. Cancer 71:2585–2597
Wong ET, Hess KR, Gleason MJ et al (1999) Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17:2572–2578
Yung WKA, Mechtler L, Gleason MJ (1991) Intravenous carboplatin for recurrent malignant gliomas: a phase II study. J Clin Oncol 9:860
Allen JC, Walker R, Luks E et al (1987) Carboplatin and recurrent childhood brain tumors. J Clin Oncol 5(3):459–463
Yung WK, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M et al (1999) Multicenter Phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. J Clin Oncol 17:2762–2771
See SJ, Levin VA, Yung A et al (2004) 13-cis-Retinoic acid in the treatment of recurrent glioblastoma multiforme. Neurooncology 6:253–258
Allen JC, Helson L (1981) High-dose cyclophosphamide chemotherapy for recurrent CNS tumors in children. J Neurosurg 55:749–756
Longee DC, Friedman HS, Albright RE, Burger PC et al (1990) Treatment of patients with recurrent gliomas with cyclophosphamide and vincristine. J Neurosurg 72:583–588
Chamberlain MC, Tsao-Wei D (2004) Recurrent glioblastoma multiforme: salvage therapy with cyclophosphamide. Cancer 100:1213–1220
Brem H, Piantadosi S, Burger PC, Walker M, Selker R et al (1995) Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. Lancet 345:1008–1012
Jaeckle KA, Hess KR, Yung A et al (2003) Phase II evaluation of temozolomide and 13-cis-retinoic acid for the treatment of recurrent and progressive malignant glioma: a North American Brain Tumor Consortium study. J Clin Oncol 21:2305–2311
Voges J, Reszka R, Grossman A, Dittmar C, Richter R et al (2003) Image-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol 54:479–487
Batchelor TT, Gilbert MR, Supko JG et al (2004) Phase 2 study of weekly irinotecan in adults with recurrent malignant glioma: final report of NABTT 97-11. Neurooncology 6:21–27
Buckner JC, Reid JM, Wright K et al (2003) Irinotecan in the treatment of glioma patients: current and future studies of the North Cancer Central Treatment Group. Cancer 97:2352–2358
Chamberlain MC (2002) Salvage chemotherapy with CPT-11 for recurrent glioblastoma. J Neurooncol 56:183–188
Cloughesy TF, Filka E, Kuhn J et al (2003) Two studies evaluating irinotecan treatment for recurrent malignant glioma using an every 3-week regimen. Cancer 97:2381–2386
Friedman HS, Petros WP, Friedman AH et al (1999) Irinotecan therapy in adults with progressive malignant glioma. J Clin Oncol 17:1516–1525
Prados MD, Yung WKA, Jaeckle KA et al (2004) Phase 1 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neurooncology 6:44–54
Prados MD, Lamborn K, Yung WKA et al (2006) A phase 2 trail of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neurooncology 82:189–193
Stark-Vance V (2005) Bevacizumab and CPT-11 in the treatment of relapsed malignant glioma. Neurooncology 7(3):369
Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF (2006) MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology 66(8):1258–1260
Vredenburgh JJ, Desjardins A, Herndon JEII et al (2007) Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 13(4):1253–1259
Vredenburgh JJ, Desjardins A, Herndon JE et al (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25(30):4722–4729
Chen C, Silverman DHS, Geist C et al (2007) Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J Clin Oncol 25(30):4714–4721
Norden AD, Young GS, Setayesh K et al (2008) Bevacizumab for recurrent malignant glioma: efficacy, toxicity and patterns of recurrence. Neurology 70:779–787
Cloughesy T, Prados MD, Mikkelsen T et al (2008) A phase 2 randomized non-comparative clinical trial of the effect of bevacizumab alone or in combination with irinotecan on 6-month progression free survival in recurrent treatment refractory glioblastoma. J Clin Oncol 26:91s (Abstract)
Kreisl TN, Kim L, Moore K et al (2009) Phase II trial of single agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:1–10
MacDonald DR, Cascino TL, Schold SC et al (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Miller RG Jr (1981) Survival analysis. Wiley, New York, pp 114–118
Pike MC (1972) Contribution to the discussion on the paper by R. Peto and J. Peto, ‘Asymptotically efficient rank invariant procedures’. J R Stat Soc Ser A 135(20):1–203
Berry G, Kitchin RM, Mock PA (1991) A comparison of two simple hazard ratio estimators based on the logrank test. Stat Med 10:749–755
Lawless JF (1982) Statistical models and methods for lifetime data. Wiley, New York, pp 345–354
Kaplan EL, Meier P (1958) Nonparametric estimation form incomplete observations. J Am Stat Assoc 53:457–481
Stupp R, Mason WP, Van Den Bent MJ et al (2004) Concomitant and adjuvant temozolomide and radiotherapy for newly diagnosed glioblastoma multiforme. Conclusive results of a randomized phase III trial by the EORTC Brain & RT Groups and NCIC Clinical Trial Groups. J Clin Oncol 22:1s
Folkman J (2006) Angiogenesis. Ann Rev Med 57:1–18
Kerbel RS (2006) Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science 312(5777):1171–1175
Semenza GL (2008) A new weapon for attaching tumor blood vessels. N Engl J Med 358(19):2066–2067
Bao S, Wu Q, Sathornsumetee S et al (2006) Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res 66(16):7843–7848
Ferrara N (2005) VEGF as a therapeutic target in cancer. Oncology 69(Suppl 3):11–16
Jain RK, Xu L (2007) αPIGF: a new kid on the antiangiogenesis block. Cell 131:443–445
Calabrese C, Poppleton H, Kocak M et al (2007) A perivascular niche for brain tumor stem cells. Cancer Cell 11(1):69–82
Duda DG, Jain RK, Willett CG (2007) Antiangiogenics: the potential role of integrating this novel treatment modality with chemoradiation for solid cancers. J Clin Oncol 25(26):4033–4042
Gilbertson RJ, Rich JN (2007) Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer 7(10):733–736
Gorski DH, Beckert MA, Jaskowiak NT et al (1999) Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res 59(14):3374–3378
Tong RT, Boucher Y, Kozin SV et al (2004) Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res 64(11):3731–3736
Jain RK (2001) Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7(9):987–989
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307(5706):58–62
Reardon DA, Wen PY, Desjardins A, Batchelor TT, Vredenburgh JJ (2008) Glioblastoma multiforme: an emerging paradigm of anti-VEGF therapy. Expert Opin Biol Ther 8(4):541–553
Fischer I, Gagner JP, Law M et al (2005) Angiogenesis in gliomas: biology and molecular pathophysiology. Brain Pathol 15(4):297–310
Jain RK, di Tomaso E, Duda DG et al (2007) Angiogenesis in brain tumors. Nat Rev Neurosci 8:610–622
Kargiotis O, Rao JS, Kyritsis AP (2006) Mechanisms of angiogenesis in gliomas. J Neurooncol 78(3):281–293
Bao S, Wu Q, McLendon R et al (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444(7120):687–688
Folkins C, Man S, Xu P et al (2007) Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res 67(8):3560–3564
Fomchenko EI, Holland EC (2006) Origins of brain tumors—a disease of stem cells? Nat Clin Pract Neurol 2:288–289
Purow B, Fine HA (2004) Antiangiogenic therapy for primary and metastatic brain tumors. Hematol Oncol Clin North Am 18:1161–1181
Stefanik DF, Fellows WK, Rizkalla LR et al (2001) Monoclonal antibodies to vascular endothelial growth factor (VEGF) and the VEGF receptor, FLT-1, inhibit the growth of C6 glioma in a mouse xenograft. J Neurooncol 55:91–100
Stefanik DF, Rizkalla LR, Soi A et al (1991) Acidic and basic fibroblast growth factors are present in glioblastoma multiforme. Cancer Res 51(20):5760–5765
Sun L, Hui AM, Su Q et al (2006) Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell 9(4):287–300
Weis SM, Cheresh DA (2005) Pathophysiological consequences of VEGF-induced vascular permeability. Nature 437:497–504
Winkler F, Kozin SV, Tong R et al (2004) Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation angiopoietin-1 and matrix metalloproteinases. Cancer Cell 6:553–563
Lamszus K, Heese O, Westphal M (2004) Angiogenesis-related growth factors in brain tumors. Cancer Treat Res 117:169–190
Yung WK et al (2000) A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer 83(5):588–593
Eremina V, Jefferson JA, Kowalewska J et al (2008) VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358(11):1129–1136
Eskens FA, Verweij J (2006) The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors: a review. Eur J Cancer 42:3127–3139
Verheul HM, Pinedo HM (2007) Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat Rev Cancer 7:475–485
Brandes AA, Scelzi E, Salmistraro G et al (1997) Incidence of risk of thromboembolism during treatment of high-grade gliomas: a prospective study. Eur J Cancer 33:1592–1596
Marras LC, Geerts WH, Perry JR (2000) The risk of venous thromboembolism is increased throughout the course of malignant glioma: an evidence-based review. Cancer 89:640–646
Semrad TJ, O’Donnell R, Wun T et al (2007) Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg l06(4):601–608
Simanek R, Vormittag R, Hassler M et al (2007) Venous thromboembolism and survival in patients with high-grade glioma. Neurooncol 9(2):89–95
Nghiemphu PL, Green RM, Pope WB, Lai A, Cloughsey TF (2008) Safety of bevacizumab for anticoagulated patients with high grade gliomas. Neurooncology Apr 24 (Epub)
Lamborn KR, Yung AWK, Chang SM et al (2008) Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neurooncology 10:162–170
Quant EC, Norden AD, Drappatz J et al (2009) Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neurooncology March 30 (Epub ahead of print)
Bokstein F, Shpigel S, Blumenthal DTl (2008) Treatment with bevacizumab and irinotecan for recurrent high-grade glial tumors. Cancer 112(10):2267–2273
Raizer JJ, Gallot L, Cohn R et al (2007) A phase II safety study of bevacizumab in patients with multiple recurrent or progressive malignant gliomas. J Clin Oncol 25(18S): 2079 (Abstract)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chamberlain, M.C., Johnston, S.K. Salvage therapy with single agent bevacizumab for recurrent glioblastoma. J Neurooncol 96, 259–269 (2010). https://doi.org/10.1007/s11060-009-9957-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-009-9957-6