Abstract
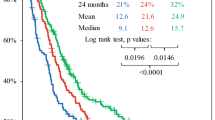
The standard of care for newly diagnosed glioblastoma multiforme (GBM) is temozolomide (TMZ) chemotherapy given concurrently with radiation for 6 weeks followed by 6 months of adjuvant TMZ. Originally, patients in Alberta were treated with only six cycles of adjuvant TMZ regardless of clinical status but institutional policy was amended to allow up to 12 cycles of adjuvant therapy for patients experiencing at least stable disease and minimal toxicity. We conducted a population-based analysis to determine if extended adjuvant TMZ treatment (i.e., more than six cycles) confers a survival advantage as compared to the standard six cycles for newly diagnosed GBM patients. Patient data was collected from the Alberta Cancer Registry and patient charts. Progression free—and overall survival was determined in patients receiving six cycles of adjuvant TMZ and compared with that of patients receiving more than six cycles. Patients in whom adjuvant chemotherapy was stopped at cycle six experienced a median survival of 16.5 months, whereas, those who received more than six cycles survived for 24.6 months (p = 0.031). Extended adjuvant therapy was not associated with increased toxicity. In multivariate analysis, adjuvant monthly Temozolomide for more than six cycles was an independent prognostic factor for both progression free—and overall survival. These data suggest extended adjuvant temozolomide (i.e., more than six cycles) should be considered in patients with newly diagnosed GBM.


Similar content being viewed by others
References
Ohgaki H, Kleihues P (2005) Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol 64:479–489
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5 year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Newlands ES, Stevens MF, Wedge SR, Wheelhouse RT, Brock C (1997) Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev 23:35–61
Hau P, Koch D, Hundsberger T et al (2007) Safety and feasibility of long-term temozolomide treatment in patients with high-grade glioma. Neurology 68:688–690
Mason WP, Maestro RD, Eisenstat D et al (2007) Canadian recommendations for the treatment of glioblastoma multiforme. Curr Oncol 14:110–117
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Hegi ME, Diserens AC, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Andre T, Louvet C, Maindrault-Goebel F et al (1999) CPT-11 (irinotecan) addition to bimonthly, high-dose leucovorin and bolus and continuous-infusion 5-fluorouracil (FOLFIRI) for pretreated metastatic colorectal cancer. GERCOR. Eur J Cancer 35:1343–1347
Brandes AA, Franceschi E, Tosoni A et al (2008) MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 26:2192–2197
Roldan GB, Scott JN, McIntyre JB et al (2009) Population-based study of pseudoprogression after chemoradiotherapy in GBM. Can J Neurol Sci 36:617–622
Sanghera P, Perry J, Sahgal A et al (2010) Pseudoprogression following chemoradiotherapy for glioblastoma multiforme. Can J Neurol Sci 37:36–42
Taal W, Brandsma D, de Bruin HG et al (2008) Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 113:405–410
Gilbert MR, Wang M, Aldape KD, et al. (2011) RTOG 0525: a randomized phase III trial comparing standard adjuvant temozolomide (TMZ) with a dose–dense (dd) schedule in newly diagnosed glioblastoma (GBM). J Clin Oncol 29:2006. (Abstract)
Acknowledgment
The authors thank Ms Qiuli Duan for her assistance with statistical analysis.
Conflict of interest
This study did not receive any corporate, institutional or governmental funding. Drs Roldán, Singh and Easaw report no disclosure.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roldán Urgoiti, G.B., Singh, A.D. & Easaw, J.C. Extended adjuvant temozolomide for treatment of newly diagnosed glioblastoma multiforme. J Neurooncol 108, 173–177 (2012). https://doi.org/10.1007/s11060-012-0826-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-012-0826-3