Abstract
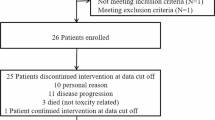
Antiangiogenic therapy can rapidly reduce vascular permeability and cerebral edema but high doses of bevacizumab may induce selective pressure to promote resistance. This trial evaluated the efficacy of low dose bevacizumab in combination with lomustine (CCNU) compared to standard dose bevacizumab in patients with recurrent glioblastoma. Patients (N = 71) with recurrent glioblastoma who previously received radiation and temozolomide were randomly assigned 1:1 to receive bevacizumab monotherapy (10 mg/kg) or low dose bevacizumab (5 mg/kg) in combination with lomustine (90 mg/m2). The primary end point was progression-free survival (PFS) based on a blinded, independent radiographic assessment of post-contrast T1-weighted and non-contrast T2/FLAIR weighted magnetic resonance imaging (MRI) using RANO criteria. For 69 evaluable patients, median PFS was not significantly longer in the low dose bevacizumab + lomustine arm (4.34 months, CI 2.96–8.34) compared to the bevacizumab alone arm (4.11 months, CI 2.69–5.55, p = 0.19). In patients with first recurrence, there was a trend towards longer median PFS time in the low dose bevacizumab + lomustine arm (4.96 months, CI 4.17–13.44) compared to the bevacizumab alone arm (3.22 months CI 2.5–6.01, p = 0.08). The combination of low dose bevacizumab plus lomustine was not superior to standard dose bevacizumab in patients with recurrent glioblastoma. Although the study was not designed to exclusively evaluate patients at first recurrence, a strong trend towards improved PFS was seen in that subgroup for the combination of low dose bevacizumab plus lomustine. Further studies are needed to better identify such subgroups that may most benefit from the combination treatment.




Similar content being viewed by others
References
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Gilbert MR, Wang M, Aldape KD et al (2013) Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 31(32):4085–4091
Wong ET, Hess KR, Gleason MJ et al (1999) Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17(8):2572–2578
Wick W, Puduvalli VK, Chamberlain MC et al (2010) Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol 28(7):1168–1174
Carmeliet P (2005) Angiogenesis in life, disease and medicine. Nature 438(7070):932–936
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307(5706):58–62
Cohen MH, Shen YL, Keegan P, Pazdur R (2009) FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist 14(11):1131–1138
Vredenburgh JJ, Desjardins A, Herndon JE 2nd et al (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25(30):4722–4729
Vredenburgh JJ, Desjardins A, Herndon JE 2nd et al (2007) Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 13(4):1253–1259
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27(28):4733–4740
Chinot OL, Wick W, Cloughesy T (2014) Bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370(21):2049
Gilbert MR, Dignam JJ, Armstrong TS et al (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370(8):699–708
Jain RK (2001) Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7(9):987–989
Winkler F, Kozin SV, Tong RT et al (2004) Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6(6):553–563
von Baumgarten L, Brucker D, Tirniceru A et al (2011) Bevacizumab has differential and dose-dependent effects on glioma blood vessels and tumor cells. Clin Cancer Res 17(19):6192–6205
de Groot JF (2011) High-dose antiangiogenic therapy for glioblastoma: less may be more? Clin Cancer Res 17(19):6109–6111
Lorgis V, Maura G, Coppa G et al (2012) Relation between bevacizumab dose intensity and high-grade glioma survival: a retrospective study in two large cohorts. J Neurooncol 107(2):351–358
Stark-Vance V (2005) Bevacizumab and CPT-11 in the treatment of relapsed malignant glioma. Paper presented at World Federation of Neuro-Oncology, Edinburgh
Ma J, Waxman DJ (2008) Combination of antiangiogenesis with chemotherapy for more effective cancer treatment. Mol Cancer Ther 7(12):3670–3684
Field KM, Jordan JT, Wen PY, Rosenthal MA, Reardon DA (2015) Bevacizumab and glioblastoma: scientific review, newly reported updates, and ongoing controversies. Cancer 121(7):997–1007
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28(11):1963–1972
Rubinstein LV, Korn EL, Freidlin B, Hunsberger S, Ivy SP, Smith MA (2005) Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol 23(28):7199–7206
Taal W, Oosterkamp HM, Walenkamp AM et al (2014) Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 15(9):943–953
Wick W, Brandes A, Gorlia T et al (2015) LB-05PHASE III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: the EORTC 26101 trial. Neurooncol 17(suppl 5):v1
Heiland DH, Masalha W, Franco P, Machein MR, Weyerbrock A (2016) Progression-free and overall survival in patients with recurrent glioblastoma multiforme treated with last-line bevacizumab versus bevacizumab/lomustine. J Neurooncol 126(3):567–575
Tonder M, Eisele G, Weiss T et al (2014) Addition of lomustine for bevacizumab-refractory recurrent glioblastoma. Acta Oncol 53(10):1436–1440
Wiestler B, Radbruch A, Osswald M et al (2014) Towards optimizing the sequence of bevacizumab and nitrosoureas in recurrent malignant glioma. J Neurooncol 117(1):85–92
Kaloshi G, Brace G, Rroji A et al (2013) Bevacizumab alone at mg/kg in an every-3-week schedule for patients with recurrent glioblastomas: a single center experience. Tumori 99(5):601–603
Desjardins A, Reardon DA, Coan A et al (2012) Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer 118(5):1302–1312
Reardon DA, Desjardins A, Peters K et al (2011) Phase II study of metronomic chemotherapy with bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. J Neurooncol 103(2):371–379
Reardon DA, Desjardins A, Peters KB et al (2012) Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naive, recurrent glioblastoma. J Neurooncol 107(1):155–164
Lu-Emerson C, Norden AD, Drappatz J et al (2011) Retrospective study of dasatinib for recurrent glioblastoma after bevacizumab failure. J Neurooncol 104(1):287–291
Funding
National Institutes of Health [1R21CA152024-01] to J. D. National Institutes of Health [CCSG-P30 CA016672] to R. D.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. W. serves on the advisory board for Actelion. X. H. has no disclosures. D. L. has no disclosures. C. C. has consultant relationships with Actelion, DNAtrix, Reata Pharma, Newlink Genetics and Cytrx Corp. M. G. has no disclosures. M. L. has no disclosures. B. O. has no disclosures. M. P-P. has no disclosures. V. P. is a consultant for Orbus Therapeutics, Foundation Medicine, Celgene, Genetech, and Merck. I. T-L. has no disclosures. R. C. has no disclosures. W. Y. is a consultant and serves on the advisory board for Actelion, DNATrix, Merck, and Novartis. J. D. serves on the advisory board for Genentech, Inc., Novartis, Celldex Therapeutics, and Foundation Medicine, Inc. J. D. serves on the DSMB for VBL Therapeutics and is a consultant for Celldex Therapeutics, OXiGENE, Omniox, Inc. and Deciphera Pharmaceuticals. J. D. receives research support from Sanofi-Aventis, AstraZeneca, EMD-Serono, Eli Lilly, Novartis, and Deciphera Pharmaceuticals.
Rights and permissions
About this article
Cite this article
Weathers, SP., Han, X., Liu, D.D. et al. A randomized phase II trial of standard dose bevacizumab versus low dose bevacizumab plus lomustine (CCNU) in adults with recurrent glioblastoma. J Neurooncol 129, 487–494 (2016). https://doi.org/10.1007/s11060-016-2195-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2195-9