Abstract
Schizophrenic patients have impairments in cognitive function, including deficits in visuospatial working memory (VSWM). VSWM is mediated, in part, by prefrontal cortical dopamine (DA) function, and dysregulation of prefrontal cortical DA systems may contribute to the pathophysiology of schizophrenia. Nicotine has complex effects on spatial working memory (SWM) in animal studies, with most studies demonstrating enhancement of SWM. Cigarette smoking is highly prevalent in schizophrenia, and these patients may smoke cigarettes to remediate cognitive deficits. The present study examined the effects of acute (<1 week) and prolonged (8–10 weeks) smoking abstinence on VSWM in schizophrenic (n = 23) and control (n = 29) nicotine-dependent cigarette smokers during placebo-controlled smoking cessation trials. Schizophrenic and control smoking patients had significant impairments in VSWM compared to non-smoking controls, after adjusting for differences in age, education and depressive symptoms. Schizophrenic smokers who quit smoking had further impairments in VSWM, and control quitters had improvements in VSWM. Abstinence-induced changes in VSWM varied as a function of gender in controls, but not in schizophrenics. These changes in VSWM appeared to be independent of study medications, and smoking abstinence did not significantly alter performance on the Stroop Color Word Test in either group. These results suggest that smoking abstinence differentially alters VSWM in schizophrenic vs. control smokers, and that cigarette smoking has beneficial effects on VSWM in schizophrenic, but not control, smokers.
Similar content being viewed by others
Main
Schizophrenic patients have high rates of cigarette smoking (58–88%) vs. 25% in the general population (Hughes et al. 1986; Ziedonis and George 1997; Dalack et al. 1998; NIDA/CPDD 1999). Numerous reasons for the high rates of cigarette smoking in these patients have been proposed including self-medication of psychopathology, shared genetic factors that confer susceptibility to both schizophrenia and nicotine dependence and environmental factors such as stress and peer modeling (Leonard et al. 1996; Dalack et al. 1998; George et al. 2000b). With respect to the self-medication hypothesis for smoking in schizophrenia, one specific reason for these high rates of cigarette smoking in these patients may be alleviation of cognitive dysfunction and the presumed hypofunctionality of cortical dopamine (DA) systems, which may be present in this illness (Knable and Weinberger 1997; Dalack et al. 1998; George et al. 1998; George et al. 2000a; George et al. 2000b).
Deficits in spatial working memory (SWM) are one of the cognitive impairments that have been consistently demonstrated in schizophrenics compared to healthy individuals (Park and Holzman 1992; Keefe et al. 1995). Recent functional neuroimaging studies have confirmed deficits in prefrontal cortical activation during performance of SWM tasks in schizophrenic vs. control subjects (Callicott et al. 1998; Manoach et al. 2000). SWM is known to be dependent in part on prefrontal cortical DA systems, and the presumed cortical DA hypofunction in schizophrenia may explain deficits in SWM in this disorder (Williams and Goldman-Rakic 1995; Zahrt et al. 1997; Castner et al. 2000). Interestingly, deficits in SWM have also been shown in patients with Parkinson's disease, which is characterized neurochemically by nigrostriatal DA depletion (Owen et al. 1993; Postle et al. 1997) suggesting that alterations in other DA pathways influence SWM function. Nicotine administration has also been shown to improve spatial working memory function in rodents (Kim and Levin 1996; Levin et al. 1999) and reverse haloperidol-induced attentional deficits in schizophrenic subjects (Levin et al. 1996). A recent study of acute cigarette smoking on SWM found that SWM was impaired by smoking in healthy control smokers (Park et al. 2000), and this paradoxical effect of cigarette smoking in control smokers may relate to the phenomenon of “proactive interference” which has been described in animals, whereby pre-exposure to nicotine results in impaired working memory task performance after subsequent nicotine exposure (Dunnett and Martel 1990). However, the effects of cigarette smoking and smoking abstinence on spatial working memory in schizophrenic patients have not been reported.
There have been several studies of the effects of nicotine administration on DA systems in rats that suggest that nicotine stimulates central DA release and metabolism (Vezina et al. 1992; Nisell et al. 1996; George et al. 1998; George et al. 2000a; George et al. 2000c), in both subcortical and cortical DA terminal fields. The few studies of nicotine withdrawal in both animals (Ward et al. 1991; Fung et al. 1996; George et al. 1998) and humans (West et al. 1984; Ward et al. 1991) have suggested that nicotine withdrawal leads to decreases in central DA (and catecholamine) function. Since SWM is dependent on central DA function, smoking abstinence may lead to alterations in SWM function in both schizophrenic and control smokers.
In the present study, we sought to compare the effects of cigarette smoking, and smoking abstinence, on visuospatial working memory (VSWM) function in patients with schizophrenia and healthy control subjects. Specific goals were: 1) to compare VSWM function in schizophrenic and control smokers, and to non-smoking schizophrenic patients and controls; and, 2) to determine the effects of acute and prolonged smoking abstinence on VSWM in schizophrenic and control smokers during the course of placebo-controlled pharmacotherapy trials.
METHODS
Subjects
Twenty-three schizophrenic and 29 healthy control smokers, and 8 schizophrenic and 16 control non-smokers, were recruited into this study. Psychiatric diagnoses were established using the Structured Clinical Interview for DSM-IV (First 1994). Schizophrenic smokers were recruited from advertisements at The Connecticut Mental Health Center for a study comparing bupropion hydrochloride vs. placebo for smoking cessation in schizophrenia. Healthy control smokers were recruited through advertisements in local newspapers for a study comparing selegiline hydrochloride vs. placebo for smoking cessation. Schizophrenic and control non-smokers were recruited by local advertisements and word of mouth. The protocols were approved by the Human Investigation Committee of Yale University School of Medicine, and informed consent for study participation was obtained for all schizophrenic and control subjects.
Procedures
The computerized neuropsychological tasks were administered at study baseline (Week 1; prior to when subjects began study medication), during Week 4 of the trial (within one week of smoking quit date) and at trial endpoint (Week 8 in controls, Week 10 in schizophrenic subjects). The procedures for the computerized visuospatial working memory (VSWM) and Stroop Color Word Test (SCWT) were adapted from previously published “pen-and-paper” versions of these tests (Keefe et al. 1995; Hepp et al. 1996), using PsyScope version 1.1 on a Macintosh computer. For both the VSWM and SCWT, subjects sat in front of the Macintosh computer with a viewing distance of 60 cm, and a visual field of ∼50 degrees. All subjects completed the VSWM task (the task of primary interest), while approximately 80% (60/76) of subjects completed the SCWT (See Figure 1).
For the VSWM task, each trial involved the presentation of three sequential screens. In Screen 1, subjects visualized a dot (1 cm in diameter) at one of 16 pre-set locations on the computer screen for a duration of three seconds. Screen 2 involved presentation of a “distractor task” screen with a sham performance task (“tic-tack-toe”) which appeared for a fixed time delay (30 seconds); subjects were instructed to indicate with the keyboard whether the game was “won” or a “draw”, and sequential “tic-tack-toe” screens appeared (with a default of five seconds if no response was received from the subject) until the 30 second delay period had elapsed. The final screen (Screen 3) involved the subjects being prompted by a question mark to identify the exact location where the object in Screen 1 appeared on the screen (default length of 5 seconds), by moving the cursor to the screen location where the subject recalled the dot was located in Screen 1. A blank screen appeared for a total of two seconds between trials. A total of 16 trials were completed, and VSWM performance results are reported as the averaged “distance from target” in cm for the 16 trials. This computerized version of the VSWM task took approximately 15 minutes to complete.
The SCWT measures participants’ ability to shift their perceptual set to conform to changing conditions, and also assesses executive functions such as mental control, response flexibility, and the occurrence of perceptual interference. Participants were required to report the color in which each word is printed, and used numbered keys on the computer keyboard (e.g. “1” for green, “2” for red, “3” for blue) to indicate color choice. Color words appeared for 500 msec. The difference in time (msec) to respond to the asynchronous color name (i.e. the word BLUE, presented in red letters) compared to the neutral condition (e.g. a series of Xs presented in red letters) is known as the Stroop Interference (SI). A total of 32 color-word pairs were presented. This computerized version of the SCWT takes approximately 15 minutes to complete.
Verification of Smoking Abstinence
At baseline, all subjects had assessment of Fagerstrom Test for Nicotine Dependence (FTND) scores (Heatherton et al. 1991), average weekly cigarettes/day smoked, expired breath carbon monoxide (CO) determination (Vitalograph CO Breathalyzer, Lenexa, KS) and plasma and urine cotinine levels (Foundation for Blood Research, Scarborough, ME). At baseline, all schizophrenic and control smokers had an FTND score >5, expired breath CO >10 ppm, self-reported smoking of at least 20 cigarettes/day in the week prior to assessment and plasma and urine cotinine levels >150 ng/ml and >600 ng/ml respectively. The smoking quit date was set at the beginning of Week 3 of each study for schizophrenic and control smokers, and all subjects whose neuropsychological data was analyzed attempted smoking abstinence at this time. Subjects were classified as abstinent from smoking if at trial endpoint (Week 10 in schizophrenics, Week 8 in controls) if: 1) they endorsed smoking abstinence, and; 2) they had an expired breath CO level <10 ppm; in some cases, smoking abstinence was confirmed with a plasma cotinine level <50 ng/ml. The Tiffany Questionnaire for Smoking Urges (QSU) (Tiffany and Drobes 1991) was used to monitor nicotine craving and withdrawal symptoms during the trials. CO levels were obtained at the time of each neuropsychological testing session.
Statistical Analysis
Analysis of covariance (ANCOVA) was used to compare baseline VSWM (“distance from target” in cm) and Stroop Interference (SI, msec) measurements among the four groups, adjusting for differences in age, education and depressive symptoms between study groups. Two- and 3-factor repeated measures analysis of variance (ANOVA) (using diagnosis, smoking status and medication assignment as between-subject factors, and time as a within-subjects factor) was used to examine differences on VSWM and SI between quitters and non-quitters in both schizophrenic and control smoker groups during the course of the smoking cessation trials. Data analysis was performed using the Statistical Package for the Social Sciences (SPSS) v10.0 software.
RESULTS
Demographic and Clinical Characteristics of Study Subjects
The demographic and clinical characteristics of schizophrenic (SCZ) and control (CON) smokers and non-smokers are given in Table 1 . SCZ and CON non-smokers significantly differed on age and years of education (p < .05). SCZ non-smokers had higher PANSS negative and total symptom scores, and lower Beck Depression Inventory (BDI) scores than SCZ smokers at baseline (p < .05). Control smokers were significantly older and less educated than CON non-smokers, and had significantly higher BDI scores than CON non-smokers (p < .05). There were no significant differences between SCZ smokers and non-smokers on medication side effect ratings. Of the SCZ non-smokers, 3/8 were nicotine-naïve, and 5/8 had been smoking abstinent for at least one year prior to assessments.
Demographic and clinical characteristics of SCZ and CON smokers, as a function of smoking status at trial endpoint, are given in Tables 2 and 3 respectively. There were no baseline differences between SCZ patients who achieved smoking abstinence for up to 10 weeks after the quit date (Week 3) and non-abstainers, with the exception that quitters had significantly lower baseline BDI scores (p < .05) than non-quitters, and that all schizophrenic quitters were prescribed atypical antipsychotic agents (p < .05) (Table 2). There were no baseline differences between control quitters and non-quitters (Table 3). Similarly, there were no baseline differences between SCZ and CON smokers prescribed active vs. placebo study medication (data not shown).
VSWM and SI in Schizophrenic vs. Control Smokers and Non-smokers
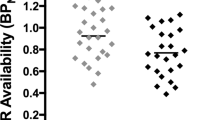
Differences in VSWM and SI as a function of psychiatric diagnosis and smoking status are shown in Figure 1. For VSWM (Top Panel), there was a significant main effect of Diagnosis (F = 26.6, 1,71, p < .01) but not Smoking Status (F = 0.31, df = 1,71, p = .58), and a significant Diagnosis X Smoking Status interaction (F = 6.85, df = 1,71, p < .05). VSWM function was significantly impaired in schizophrenic compared to control smokers (p < .05). Furthermore, schizophrenic smokers appeared to have improved VSWM function compared to non-smoking schizophrenics, but this difference was not significant (p = .20). Interestingly, healthy smokers had impairments in VSWM compared to non-smoking controls (p < .05). There were differences between groups insofar as age, education and depressive symptoms between subjects in the four groups (see Table 1), but a one-way ANCOVA adjusting for these covariates confirmed the Diagnosis X Smoking Status interaction on VSWM (F = 5.08, df = 3,69, p < .01).
For the SCWT (Bottom Panel), there was a main effect of Diagnosis (F = 9.74, df = 1,42, p < .01) on Stroop Interference (SI), with schizophrenic patients (irrespective of smoking status) having higher SI than controls (p < .05), but no main effects of Smoking Status (F = 2.24, df = 1,42, p = .14), and no Diagnosis X Smoking Status interaction (F = 0.29, df = 1,42, p = .60).
Smoking Abstinence in Schizophrenic and Control Subjects at Trial Endpoint
A total of 8/23 (34.8%) schizophrenic subjects and 11/29 (37.9%) control subjects met criteria for smoking abstinence at trial endpoint. Trial endpoint smoking abstinence rates by medication status were: Bupropion 6/12 (50.0%) vs. Placebo 2/11 (18.2%; chi-square = 2.56, df = 1, p = .11); Selegiline 8/14 (57.1%) vs. Placebo 3/15 (20.0%; chi-square = 2.89, df = 1, p = .09). Five of eight schizophrenic and 6/11 control quitters achieved continuous smoking abstinence from the quit date (Week 3) until trial endpoint. However, the directional changes in VSWM function in schizophrenic and control subjects were similar between continuous and non-continuous abstainers (see next section).
Effects of Smoking Abstinence on VSWM and SCWT Performance in Schizophrenics vs. Controls
VSWM
Smoking abstinence-related changes in VSWM in schizophrenic and control subjects are shown in Figures 2 and 3 respectively. Smoking abstinence leads to: 1) further impairment of baseline deficits in VSWM in schizophrenic smokers as evidenced by a significant Smoking Status X Time interaction (F = 6.11, df = 2,22, p < .01) (Figure 2), and; 2) improvement in VSWM in control smokers (F = 3.16, df = 2,40, p < .05) (Figure 3), confirming Diagnosis X Smoking Status interactions suggested by our between-groups data (see Figure 1). Furthermore, a repeated measures ANOVA procedure demonstrated a significant Diagnosis X Smoking Status X Time interaction for the combined VSWM data in schizophrenic and control smokers (F = 3.70, df = 2,62, p < .05). When effects of smoking abstinence on VSWM were assessed as a function of gender, we observed that there were no differences in abstinence-induced impairments in VSWM in male vs. female schizophrenic smokers, but that improvements in VSWM induced by smoking abstinence in controls were confined to female subjects. A 2-factor repeated measures ANOVA found a nearly significant Smoking Status X Gender X Time interaction (F = 3.53, dfn = 1,21, p = .07), and a significant post-hoc difference (p < .05) in VSWM between female and male control quitters at Week 8 (data not shown).
A comparison of VSWM at baseline (Week 1) compared to Week 4 and Week 10 in schizophrenic smokers treated with bupropion or placebo who did not achieve smoking abstinence suggested that there were no significant effects of bupropion on VSWM function in schizophrenic smokers (Bupropion Non-Quitter Group (n = 6), Week 1: 4.73 ± 1.02; Week 4: 5.44 ± 1.77; Week 10; 5.69 ± 2.14 cm; Placebo Non-Quitter Group (n = 9), Week 1: 6.20 ± 3.06; Week 4: 6.15 ± 2.08; Week 10: 5.76 ± 2.81; Medication X Time, F = 2.34. df = 2,24, p = .12), suggesting that the changes in VSWM with bupropion were most likely due to the effects of smoking abstinence in these patients, and were independent of study medications. In fact, the two schizophrenic subjects who achieved smoking abstinence in the placebo group had further impairments in VSWM during the trial (data not shown), and, in the total schizophrenic smoker sample, there were no significant Medication effects (F = 0.48, df = 3,13, p = .70) or Medication X Time interactions (F = 0.34, df = 2,26, p = .71) on VSWM in schizophrenic subjects. Similarly, improvements in VSWM in control smokers during smoking abstinence were likely due to the effects of smoking abstinence, and not due to the effects of the study medication (Selegiline Non-Quitter Group (n = 6), Week 1: 4.45 ± 2.46; Week 4: 5.76 ± 2.94; Week 8: 5.77 ± 2.49 cm; Placebo Non-Quitter Group (n = 12), Week 1: 3.61 ± 1.59; Week 4: 3.00 ± 2.29; Week 8: 3.43 ± 1.44; Medication X Time, F = 1.65, df = 2,32, p = .21). Three control subjects on placebo who quit smoking had the expected improvements in VSWM (data not shown), and there were no significant Medication effects (F = 1.84, df = 3,20, p = .17) or Medication X Time interactions (F = 2.27, df = 2,40, p = .12) in the total sample of control smokers. We also examined Medication X Smoking Status X Time effects on VSWM. In both schizophrenic (F = 0.41, df = 2,26, p = .66) and control (F = 0.37, df = 2,40, p = .65) groups, these interactions were not significant, but given the small group sizes for placebo quitters (n = 2 for schizophrenics, n = 3 for controls), our analysis had limited statistical power. Furthermore, sustained changes in VSWM at trial endpoint in both schizophrenic and control quitters were not due to elevated nicotine craving and withdrawal symptom ratings, since there were reductions in nicotine craving and withdrawal scores on the Tiffany QSU at trial endpoint compared to baseline (Week 1) and one-week post quit date (Week 4) ratings (data not shown).
SCWT
The effects of smoking abstinence on SCWT performance in schizophrenic and control smokers are presented in Figure 4. Schizophrenic smokers exhibited significant increases in baseline SI reaction times (Figure 4, Top Panel) compared to control (Figure 4, Bottom Panel) smokers, but there were no significant Smoking Status X Time interactions in either schizophrenic (F = 0.31, df = 2,22, p = .67) or control (F = 0.23, df = 2,34, p = .77) smokers, suggesting that smoking abstinence did not significantly alter SI. An examination of Figure 4 suggested that smoking abstinence increased SI in schizophrenic smokers, and while non-significant, this difference appeared to be due to an increase in the incongruent reaction time (data not shown). There were practice effects (decreases in SI) with repeated SCWT administration in schizophrenic vs. control patients (Figure 4). Furthermore, there were no significant effects of Medication (Schizophrenic: F = 1.22, df = 3,11, p = .35, Control: F = 1.34, df = 3,17, p = .29), Medication X Time interactions (Schizophrenic: F = 0.81, df = 2,22, p = .46, Control: F = 0.11, df = 2,34, p = .90) or Medication X Smoking Status X Time interactions (Schizophrenic: F = 0.01, df = 2,22, p = .97, Control: F = 0.34, df = 2,34, p = .69) on SI in either schizophrenic or control smokers.
DISCUSSION
Our preliminary data suggests that smoking abstinence leads to further impairment in VSWM function in schizophrenic patients, but, in contrast, produces improvements in VSWM in control smokers. The effects of smoking abstinence on VSWM appear to be independent of the study medications (e.g. bupropion and selegiline) used for smoking cessation, and nicotine craving and withdrawal symptoms. This apparent lack of effect of bupropion on VSWM in our study is of particular interest since it has been shown that this agent is: 1) a catecholamine reuptake inhibitor (Ascher et al. 1995), and; 2) a non-competitive antagonist of nicotinic acetylcholine receptor sites (Slemmer et al. 2000). One potential explanation for our findings with respect to prolonged smoking abstinence and changes in VSWM is that smoking abstinence led to increases in plasma antipsychotic levels, since cigarette smoking is known to increase the hepatic clearance of certain antipsychotic agents (Perry et al. 1993; Ziedonis and George 1997). This possibility appears unlikely since changes in VSWM in response to smoking abstinence occurred within 1 week of abstinence, and the time course for the increase in antipsychotic plasma levels after smoking cessation is in the range of 2–4 weeks (Perry et al. 1993; Ziedonis and George 1997). Furthermore, it should be noted that neither the antipsychotic medication or dose was changed during the smoking cessation trial in schizophrenic smokers, so changes in VSWM observed in schizophrenic subjects during smoking abstinence were not likely due to antipsychotic medication effects, and were probably related to the effects of smoking abstinence. We also observed that all schizophrenic quitters (n = 8) were prescribed atypical antipsychotic agents (see Table 2), suggesting that atypical antipsychotic agents may enhance smoking cessation responses to bupropion (Head et al. 2001).
The effects of atypical antipsychotic drugs on spatial working memory deficits in schizophrenic patients have not been well-characterized (Meltzer et al. 1999; Meltzer and McGurk 1999). Clozapine is known to reverse drug-induced spatial working memory deficits in non-human primates (Jentsch et al. 1997; Murphy et al. 1997), and risperidone may modestly improve SWM deficits in schizophrenic patients (McGurk et al. 1996). While differences in the rates of prescription of atypical vs. typical antipsychotic drugs between the schizophrenic smoker and schizophrenic non-smoker groups could account for some of the differences (albeit non-significant) in VSWM between these groups (Figure 1), and in comparison to the control groups, they would not account for the differences in VSWM in schizophrenic smokers who achieved smoking abstinence vs. those who did not quit smoking (e.g. within-subjects comparison; Figure 2) since antipsychotic medications were not changed during the smoking cessation intervention.
Interestingly, Park et al. (2000) recently showed that acute cigarette smoking impairs spatial working memory function (but improves spatial selective attention) in healthy smokers. Thus, it appears that smoking may have differential effects in schizophrenic and healthy control smokers, and our results may support a “self-medication hypothesis” for cigarette smoking and cognitive dysfunction in schizophrenia. VSWM is dependent, in part, on cortical dopamine (DA) function that is presumed to be reduced in schizophrenia (Williams and Goldman-Rakic 1995). Furthermore, Parkinson's disease, which is characterized by nigrostriatal DA depletion, is also associated with impairments in SWM (Owen et al. 1993; Postle et al. 1997). Thus, our data is consistent with the notion that cigarette smoking may increase cortical DA hypofunction towards “normal” levels, thereby improving VSWM in schizophrenia. In contrast, cigarette smoking may augment normal cortical DA in control smokers to excessive levels, thus impairing VSWM. This is consistent with animal data that suggests an “inverted-U” shaped response between cortical DA function and SWM (Zahrt et al. 1997; George et al. 1998; George et al. 2000b; George et al. 2000c). Furthermore, our findings in schizophrenic smokers are consistent with the results of Kirrane et al. (2000) who observed that administration of the psychostimulant D-amphetamine improves deficits in VSWM function in schizophrenic spectrum personality disorders subjects, who may have cortical DA deficits that resemble those in schizophrenic patients.
We also observed that abstinence-induced changes in VSWM varied as a function of gender in controls (e.g., the improvements in VSWM in control quitters appeared to be confined to females). However, no gender differences in VSWM in response to smoking abstinence were observed in schizophrenic smokers, consistent with the lack of gender effects on cognitive dysfunction in schizophrenic patients (Goldberg et al. 1995). Our data in control smokers suggests that there may be gender-related differences in the effects of cigarette smoking on this aspect of cognitive function. This observation could relate to estrogen's potentiation of central DA function (Castner et al. 1993; Markowska 1999).
Differences in VSWM among the four groups (SCZ and CON smokers and respective non-smoking controls) are consistent with the smoking abstinence data from the within-subjects assessments, but are complicated by differences between the smoking and non-smoking groups, including age, educational attainment and depressive symptom ratings. The significantly higher negative symptom scores, and lower depression rating scores in schizophrenic non-smokers are consistent with greater chronicity of schizophrenic illness in this group (Kirkpatrick et al. 1995). It is unclear if non-smoking schizophrenics have some trait difference compared to schizophrenic smokers, or are incomplete responders to antipsychotic drug therapy. Further controlled evaluations of clinical and cognitive measures in schizophrenic smokers and non-smokers after careful matching on baseline measures is warranted, and should clarify these issues.
Our data with administration of the SCWT suggests that schizophrenic smokers have deficits in baseline SI compared to control smokers, consistent with previous studies (Hepp et al. 1996). In the present study, smoking abstinence does not appear to significantly alter Stroop Interference in schizophrenic or control smokers, though our sample size was smaller than with the VSWM task, and thus our ability to detect significant differences between abstinent and smoking SCZ and CON groups may have been limited. Previous studies of smoking abstinence effects on SCWT have shown increases in congruent and incongruent color naming reaction times but no significant changes in SI, consistent with our study results (Provost and Woodward 1991), while administration of nicotine gum has been shown to decrease SI in nicotine-naïve, non-smoking subjects (Provost and Woodward 1991).
Limitations of this study include: 1) the confounding influence of the two study medications and the small sample of subjects in the placebo groups who quit smoking (n = 2 in schizophrenic, and n = 3 in control smoking groups), limiting our ability to analyze Medication X Smoking Status X Time interactions (though our analysis suggests that both bupropion and selegiline did not significantly alter VSWM performance in both schizophrenic and control non-quitters); 2) failure of all subjects to attain continuous smoking abstinence (as documented by CO levels) from the quit date through trial endpoint in both schizophrenic and control subjects which may have influenced the VSWM data towards a reduced difference between abstinent vs. non-abstinent SCZ and CON smoking groups; 3) demographic and clinical differences between the study groups (e.g. age, education and depressive symptoms) which could account for some of the observed baseline differences in VSWM, and; 4) no assessment of acute smoking abstinence (<24 hours) effects on VSWM function in schizophrenic vs. control groups which could more clearly indicate the onset of abstinence-related changes in VSWM function in schizophrenic vs. control smokers.
Taken together, the present results have implications for understanding the effects of cigarette smoking and nicotine on VSWM, and suggest that cigarette smoking may have beneficial effects on VSWM function in schizophrenic, but not control, smoking subjects. Our results may have implications for the development of novel treatment approaches based on nicotinic receptor mechanisms for neuropsychiatric disorders characterized by neurocognitive dysfunction, including schizophrenia and other nicotine-responsive neuropsychiatric disorders (e.g., Parkinson's Disease, Tourette's Syndrome) (Piasecki and Newhouse 2000).
References
Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, Golden RN, Martin P, Potter WZ, Richelson E . (1995): Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry 56: 395–401
Callicott JH, Ramsey NF, Tallent K, Bertolino A, Knable MB, Coppola R, Goldberg T, van Gelderen P, Mattay VS, Frank JA, Moonen CT, Weinberger DR . (1998): Functional magnetic resonance imaging brain mapping in psychiatry: methodological issues illustrated in a study of working memory in schizophrenia. Neuropsychopharmacology 18: 186–196
Castner SA, Xiao L, Becker JB . (1993): Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Research 610: 127–134
Castner SA, Williams GV, Goldman-Rakic PS . (2000): Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science 287: 2020–2022
Dalack GW, Healy DJ, Meador-Woodruff JH . (1998): Nicotine dependence and schizophrenia: clinical phenomenon and laboratory findings. Am J Psychiatry 155: 1490–1501
Dunnett SB, Martel FL . (1990): Proactive interference effects on short-term memory in rats: I. Basic parameters and drug effects. Behavioral Neuroscience 104: 655–665
First MB . (1994): Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) Washington, DC, American Psychiatric Association
Fung YK, Schmid MJ, Anderson TM, Lau Y-S . (1996): Effects of nicotine withdrawal on central dopaminergic systems. Pharmacol Biochem Behav 53: 635–640
George TP, Verrico CD, Roth RH . (1998): Effects of repeated nicotine pre-treatment on mesoprefontal dopaminergic and behavioral responses to acute footshock stress. Brain Research 801: 36–49
George TP, Verrico CD, Xu L, Roth RH . (2000a): Effects of repeated nicotine administration and footshock stress on rat mesoprefrontal dopamine systems: evidence for opioid mechanisms. Neuropsychopharmacology 23: 79–88
George TP, Zeidonis DM, Feingold A, Pepper WT, Satterburg CA, Winkel J, Rounsaville BJ, Kosten TR . (2000b): Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. American Journal of Psychiatry 157: 1835–1842
George TP, Verrico CD, Roth RH . (2000c): Nicotinic modulation of mesoprefrontal dopamine systems: Pharmacologic and neuroanatomic characterization. J Pharmacol Exp Ther 295: 58–66
Goldberg TE, Gold JM, Torrey EF, Weinberger DR . (1995): Lack of sex differences in the neuropsychological performance of patients with schizophrenia. Am J Psychiatry 152: 883–888
Head CA, Vessicchio JC, Termine A, Sahady DM, Kosten TR, George TP . (2001): Bupropion versus placebo for smoking cessation in schizophrenia: Effects of atypical antipsychotic drugs Society for Research on Nicotine and Tobacco, Seattle, WA
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO . (1991): The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addictions 86: 1119–1127
Hepp H, Maier S, Hermle L, Spitzer M . (1996): The stroop effect in schizophrenic patients. Schizophrenia Research 22: 187–195
Hughes JR, Hatsukami DK, Mitchell JE, Dahlgreen LA . (1986): Prevalence of smoking among psychiatric outpatients. American Journal of Psychiatry 143: 993–997
Jentsch JD, Redmond Jr DE, Elsworth JD, Taylor JR, Youngren KD, Roth RH . (1997): Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science 277: 953–955
Keefe RSE, Lees Roitman SE, Harvey PD, Blum CS, DuPre RL, Prieto RM, Davidson M, Davis KL . (1995): A pen-and-paper human analogue of a monkey prefrontal cortex activation task: spatial working memory in patients with schizophrenia. Schizophrenia Research 17: 25–33
Kim JS, Levin ED . (1996): Nicotinic, muscarinic and dopaminergic actions in the ventral hippocampus and the nucleus accumbens: effects on spatial working memory in rats. Brain Research 725: 231–240
Kirkpatrick B, Buchanan RW, Breier A, Carpenter Jr WT . (1995): Depressive symptoms and the deficit syndrome of schizophrenia. J Nerv Mental Dis 182: 452–455
Kirrane RM, Mitropoulou V, Nunn M, New AS, Harvey PD, Schopick F, Silverman J, Siever LJ . (2000): Effects of amphetamine on visuospatial working memory performance in schizophrenia spectrum personality disorder. Neuropsychopharmacology 22: 14–18
Knable MB, Weinberger DR . (1997): Dopamine, the prefrontal cortex and schizophrenia. J Psychopharmacol 11: 123–131
Leonard S, Adams C, Breese CR, Adler LE, Bickford P, Byerley W, Coon H, Griffith JM, Miller C, Myles-Worsley M, Nagamoto HT, Rollins Y, Stevens KE, Waldo M, Freedman R . (1996): Nicotinic receptor function in schizophrenia. Schizophrenia Bulletin 22: 431–445
Levin ED, Wilson W, Rose J, McEvoy J . (1996): Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology 15: 429–436
Levin ED, Christopher NC, Weaver T, Moore J, Brucato F . (1999): Ventral hippocampal ibotenic acid lesions block chronic nicotine-induced spatial working memory improvement in rats. Cognitive Brain Research 7: 405–410
Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL . (2000): Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biological Psychiatry 48: 99–109
Markowska AL . (1999): Sex dimorphisms in the rate of age-related decline in spatial working memory: relevance to alterations in the estrous cycle. J Neurosci 19: 8122–8133
McGurk SR, Green MG, Wirshing WC, Ames D, Marshall BD, Marder SM . (1996): The effects of risperidone versus haloperidol on spatial working memory in treatment-resistant schizophrenia. Paper presented at 51st Annual Meeting of the Society for Biological Psychiatry, New York, NY
Meltzer HY, Park S, Kessler R . (1999): Cognition, schizophrenia, and the atypical antipsychotic drugs. Proc Natl Acad Sci USA 96: 13591–13593
Meltzer HY, McGurk SR . (1999): The effects of clozapine, risperidone and olanzapine on cognitive function in schizophrenia. Schizophrenia Bulletin 25: 233–255
Murphy BL, Roth RH, Arnsten AF . (1997): Clozapine reverses the spatial working memory deficits induced by FG7142 in monkeys. Neuropsychopharmacology 16: 433–437
NIDA/CPDD. (1999): Consensus Statement on Evaluation of Outcomes for Pharmacotherapy of Substance Abuse/Dependence. F Vocci, DeWit H (eds.). Washington, DC
Nisell M, Nomikos GG, Hertel P, Panagis G, Svennson TH . (1996): Condition-independent sensitization of locomotor stimulation and mesocortical dopamine release following chronic nicotine treatment in the rat. Synapse 22: 369–381
Owen AM, Beksinska M, James M, Leigh PN, Summers BA, Marsden CD, Quinn NP, Sahakian BJ, Robbins TW . (1993): Visuospatial working memory at different stages of Parkinson's disease. Neuropsychologia 31: 627–644
Park S, Holzman PS . (1992): Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry 49: 975–982
Park S, Knopnick C, McGurk S, Meltzer HY . (2000): Nicotine impairs spatial working memory while leaving spatial attention intact. Neuropsychopharmacology 22: 200–209
Perry PJ, Miller DD, Arndt SV, Smith DA, Holman TL . (1993): Haloperidol dosing requirements: The contributions of smoking and nonlinear pharmacokinetics. J Clin Psychopharmacol 13: 46–51
Piasecki M, Newhouse PA . (2000): Nicotine in Psychiatry: Psychopathology and Emerging Therapeutics. Washington, DC, American Psychiatric Press, Inc
Postle BR, Jonides J, Smith EE, Corkin S, Growdon JH . (1997): Spatial, but not object, delayed response is impaired in early Parkinson's disease. Neuropsychology 11: 171–179
Provost SC, Woodward R . (1991): Effects of nicotine gum on repeated administration of the Stroop test. Psychopharmacology 104: 536–540
Slemmer JE, Martin BR, Damaj MI . (2000): Bupropion is a nicotinic antagonist. J Pharmacol Exp Therap 295 (1): 321–327
Tiffany ST, Drobes DJ . (1991): The development and initial validation of a questionnaire on smoking urges. Addiction 86: 1467–1476
Vezina P, Blanc G, Glowinski J, Tassin JP . (1992): Nicotine and morphine differentially activate brain dopamine in prefrontalcortical and subcortical terminal fields: effects of acute and repeated injections. JPET 261: 484–490
Ward KD, Garvey AJ, Bliss RE, Sparrow D, Young JB, Landsberg L . (1991): Changes in urinary catecholamine excretion after smoking cessation. Pharmacol Biochem Behav 40: 937–940
West RJ, Russell MAH, Jarvis MJ, Pizzey T, Kadam B . (1984): Urinary adrenaline concentrations during 10 days of smoking abstinence. Psychopharmacology 84: 141–142
Williams GV, Goldman-Rakic PS . (1995): Modulation of memory fields by dopamine D1 receptors in the prefrontal cortex. Nature 376: 572–575
Zahrt J, Taylor JR, Matthew RG, Arnsten AF . (1997): Supranormal stimulation of D1 receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci 17: 8528–8535
Ziedonis DM, George TP . (1997): Schizophrenia and nicotine use: report of a pilot smoking cessation program and review of neurobiological and clinical issues. Schizophrenia Bulletin 23: 247–254
Acknowledgements
This work was supported in part by USPHS grants K12-DA-00167 and R01-DA-14039 (to TPG), P50-DA-12762 (TRK), P50-DA-13334 (to S.S. O'Malley), a VISN 1 Mental Illness Research, Education and Clinical Care Center from the U.S. Department of Veteran Affairs (to B.J. Rounsaville), and a Wodecroft Foundation Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression (NARSAD) to T.P.G. We thank Alan Feingold, Ph.D. for statistical assistance, and Cheryl A. Satterburg, M.S.W., Xavier D. Lara, M.D. and Thomas A. Bregartner, B.A. for assistance with the clinical and neuropsychological assessments used in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
George, T., Vessicchio, J., Termine, A. et al. Effects of Smoking Abstinence on Visuospatial Working Memory Function in Schizophrenia. Neuropsychopharmacol 26, 75–85 (2002). https://doi.org/10.1016/S0893-133X(01)00296-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(01)00296-2
Keywords
This article is cited by
-
The negative impact of chronic tobacco smoking on adult neuropsychological function: a cross-sectional study
BMC Public Health (2021)
-
Traits and Biomarkers for Addiction Risk in Schizophrenia
Current Addiction Reports (2017)
-
Translating Neurobiology to the Treatment of Dual Diagnosis: The Example of Nicotinic Receptors and Neurocognitive Endophenotypes in Schizophrenia
Current Addiction Reports (2014)
-
Electronic nicotine delivery systems for smoking cessation: where are we?
Current Respiratory Care Reports (2014)
-
Smoking improves divided attention in schizophrenia
Psychopharmacology (2014)