Abstract
In 112 schizophrenic patients previously treated with typical neuroleptics, we investigated the putative role of the dopamine D3 receptor gene (DRD3) in tardive dyskinesia (TD). Patients were assessed for TD severity using the Abnormal Involuntary Movement Scale (AIMS) and were subsequently genotyped for the MscI polymorphism that identifies a serine to glycine substitution in DRD3. A modified analysis of covariance model, which incorporated several clinical risk factors for TD, was utilized to detect differences in TD severity among the various genotypic groups. The glycine allele of DRD3 was found to be associated with typical neuroleptic-induced TD (F[2,95] = 8.25, p < .0005). Higher mean AIMS scores were found in patients homozygous for the glycine variant of the DRD3 gene, as compared to both heterozygous and serine homozygous patients. Although replication is necessary, this finding supports a role for the dopamine D3 receptor in the pathogenesis of TD.
Similar content being viewed by others
Main
Schizophrenia is a common and devastating neuropsychiatric syndrome afflicting approximately 1% of the human population (APA 1994). Although there are no cures, neuroleptic medications are frequently used to alleviate the psychosis. One of the major drawbacks to chronic treatment with typical neuroleptic drugs is the possible development of severe extrapyramidal side effects, such as pseudoparkinsonism, acute dystonia, akathisia, and tardive dyskinesia (TD). TD is a motor disorder characterized by abnormal involuntary movements of the orofacial musculature (Caligiuri et al. 1988), particularly in the jaw, lips, and tongue. Choreoathetoid movements of the extremities and/or trunk involvement may occur as well (Weinhold et al. 1981). TD occurs in predisposed individuals during or following cessation of prolonged typical neuroleptic treatment (Gerlach and Casey 1984). Schooler and Kane (1982) outline various subtypes of TD, including probable TD, masked TD, transient TD, withdrawal TD, persistent TD, and masked persistent TD. Atypical antipsychotics have a lower occurrence of extrapyramidal side effects and rarely cause TD. Recent studies have shown that clozapine may ameliorate TD symptoms (Buchanan 1995; Kane et al. 1993; Lieberman et al. 1991; Ranjan and Meltzer 1996; Wilson 1992). However, clozapine's use is limited by its lack of efficacy in some patients and its propensity to induce agranulocytosis. Although the mechanism of TD remains unknown, it has been postulated that an overactivity of dopaminergic neurotransmission in the basal ganglia may play a crucial role in the manifestation of TD (Tarsy and Baldessarini 1977).
The dopamine overactivity hypothesis for TD is supported by the clinical pharmacology of TD, because dopamine agonists increase TD severity, whereas, dopamine antagonists reduce the symptoms (Wolfe and Mosnaim 1988). The hypothesis speculates that TD results from chronic neuroleptic blockade of dopamine D2-like receptors in the basal ganglia, and states that long-term blockade results in an upregulation of dopamine receptors on postsynaptic membranes (Casey et al. 1982; Gerlach and Casey 1984; Meshul and Casey 1989). It has been suggested that the neuroleptic-induced increase in dopamine receptor density eventually results in an overactivity of the nigrostriatal dopaminergic tract (Gordon and Fields 1989), a brain region involved in the regulation of motor behavior. Despite the apparent support of the dopamine receptor upregulation hypothesis of TD, studies have failed to provide conclusive evidence supporting its role in the mechanism of TD. More recent studies have adopted a hypothesis of multiple neurotransmitter system involvement (Jeste and Caligiuri 1993).
In the treatment of schizophrenia with typical neuroleptics, approximately 20–30% of patients suffer from neuroleptic-induced TD (Awouters et al. 1990; Casey 1991; Hoffman et al. 1987; Jeste and Caligiuri 1993; Lieberman et al. 1988), thus implying variable susceptibility to the disorder. It is quite possible that genetic variability may account for some of this susceptibility and/or protection, predisposing only certain patients to TD. Animal models also suggest that there is interindividual susceptibility to TD. In the Cebus monkey, even after several years of neuroleptic administration, only some develop this disorder (Casey 1992). This is also evident in rat models of TD in which rats have been shown to dichotomize into two groups either expressing or not expressing vacuous chewing movements (VCM)(Hashimoto et al. 1998). Another rat model of TD, characterized by repetitive jaw movements (RJM), can be induced by neuroleptic drugs and has been used to study the dopamine overactivity hypothesis by Rosengarten et al. (1993a,b). They found that by selectively inbreeding rats, they were able to produce rats that differed in RJM responses, and they postulate further that genetic factors may play a role in TD patients treated with neuroleptics (Rosengarten et al. 1994). Recent studies have analyzed the roles of opioid receptors (Sasaki et al. 1996) and GABA receptors (Sasaki et al. 1997) in haloperidol-induced rat models of TD. These studies all support the hypothesis that TD may be partially controlled by genetic factors.
A genetic predisposition to TD in humans has been suggested (O'Callaghan et al. 1990; Rosengarten et al. 1993a, b; Swartz et al. 1997), and the observation that TD is more likely to occur in patients with a strong familial history of psychiatric disorder provides further evidence for the involvement of genetic factors (Guala et al. 1992; O'Callaghan et al. 1990; Waddington and Youssef 1988). In terms of familial aspects of TD, it was found that eight patients and their first degree relatives showed concordance for the presence or absence of TD (Yassa and Ananth 1981). Concordance for TD has also been noted in two brothers with schizophrenia (Weinhold et al. 1981) and in six other pairs; each of two brother–brother pairs; three brother–sister pairs, and one mother–daughter pair (Youssef et al 1989). Also, concordance for TD was observed in four siblings with schizophrenia (Waddington and Youssef 1988). A recent preliminary study examined 16 relative pairs for the presence or absence of TD and found concordance for TD in 13 of these relative pairs (Muller et al. 1998). Overall, this evidence supports the role of genetic factors in the manifestation of TD.
In terms of candidate genes, the D2 receptor gene was initially thought to be the most likely candidate involved in TD. Chen and colleagues (1997) reported a significant association between the dopamine D2 receptor gene (DRD2) and TD; however, Inada et al. (1997) did not find any association between DRD2 and TD. Our analysis of DRD2 and TD revealed no significant effect (Badri et al. 1996). One particular candidate from the D2-like receptor family, which has not received much research interest in terms of TD, is the dopamine D3 receptor. Autoradiographic studies, as well as histochemical studies, have localized dopamine D3 mRNA and protein to the ventral side of the striatum and the ventral putamen in the basal ganglia, a brain region implicated in motor control (Joyce and Meador-Woodruff 1997; Suzuki et al. 1998). A study by Buckland and McGuffin (1992) demonstrated that D3 mRNA levels in rat brain increase following chronic administration of haloperidol. A human postmortem study has recently illustrated a 45 to 56% increase in the number of D3 receptors in the basal ganglia of neuroleptic-treated schizophrenics, as compared to controls (Meador-Woodruff et al. 1997). A more recent study revealed a twofold increase in the number of D3 receptors in the basal ganglia of long-term hospitalized patients with schizophrenia when compared with matched controls (Gurevich et al. 1997). Furthermore, pharmacological studies provide evidence that dopamine D3 receptors provide an inhibitory effect on locomotor activity. A study by Kling-Petersen and colleagues (1995), demonstrated that R-(+)-7-OH-DPAT, a dopamine D3 preferring agonist, inhibited locomotor activity when injected into the nucleus accumbens of rat brain. They also showed that dopamine D3 antagonists resulted in an increase in locomotor activity. Several other studies have demonstrated that dopamine D3 preferring agonists inhibit locomotor activity; whereas, dopamine D3 antagonists increase locomotor activity (Fink-Jensen et al. 1998; Gendreau et al. 1997; Van Hartesveldt 1997). These pharmacological results are in accordance with a recent study that looked at locomotor behavior in mice lacking functional dopamine D3 receptors. D3 knock-out mice displayed increased locomotor activity and were considered hyperactive (Accili et al. 1996). Thus, evidence suggests that the dopamine D3 receptor may be involved in motor control.
This study investigates the putative role of the Ser9Gly polymorphism of the dopamine D3 receptor (DRD3) gene, as a predisposing factor to neuroleptic-induced TD. This polymorphism is interesting because it identifies a serine to glycine amino acid substitution in the N-terminal extracellular domain of the D3 receptor (Lannfelt et al. 1992), and a recent functional study of the Ser9Gly polymorphism in CHO cells revealed allelic differences in affinity for dopamine (Lundstrom and Turpin 1996). Following our initial report (Badri et al. 1996, Steen and colleagues (1997) have reported a relationship between the dopamine D3 receptor gene and TD. A preliminary report by Macciardi and colleagues (1996) also demonstrated an association between DRD3 and TD. However, Inada and colleagues (1997) did not find any association between DRD3 and TD. Gaitonde et al. (1996) studied general movement disorders and investigated the Ser9Gly polymorphism using an over-all movement disorder score and also found no association. In pursuit of the dopamine hypothesis of TD and as a follow up to our initial report of association between DRD3 and TD (Badri et al. 1996), we explore the possible etiologic role of DRD3 in TD. Our study was designed to evaluate the relationship between neuroleptic induced TD and the Ser9Gly genetic polymorphism of the DRD3 gene and is, to our knowledge, the first to use a more powerful parametric statistic that controls for clinical factors, including ethnicity, age, and sex. We hypothesize that a particular variant of the Ser9Gly polymorphism of DRD3 will be associated with the severity of typical neuroleptic-induced TD. Identification of DRD3 as a genetic risk factor for TD should aid in the elucidation of possible biological mechanisms underlying the disorder's pathogenesis and may be useful in determining effective pharmacotherapy.
METHODS
Clinical Sample
112 patients (81 male and 31 female) with DSM-IIIR diagnoses of schizophrenia and who were either treatment-refractory or intolerant to typical antipsychotic therapy (Kane et al. 1988a), were recruited from the following independent research clinics: Case Western Reserve University in Cleveland (HY Meltzer, n=74); Hillside Hospital in Long Island (JA Lieberman, n=25); and the University of California at Irvine (SG Potkin, n=13). Written informed consent was obtained from each patient. Patient age ranged from 16 to 58 (mean = 32.9, SD = 9.6), and the average duration of illness was 13.7 years (SD = 9.2). In terms of ethnicity, 75.9% of the patients were Caucasian, 1.8% were Asian, and the remaining 22.3% were African American. Ethnicity was determined using a form filled out by the clinician for each patient. Place of birth for each patient, their parents and grandparents, as well as mother tongue and religion were used to assess ethnic status. DRD3 allelic frequency differences were assessed between Caucasians and African Americans using the chi-square statistic. Table 1 summarizes the demographic distribution of the patients from each of the clinical sites. Although full neuroleptic histories were not available for all patients, it was known that all had been treated with typical neuroleptics from at least two chemical classes for a minimum of 1 year. Patients received at least three periods of treatment in the preceding 5 years at doses equivalent to or greater than 1000 mg/d of chlorpromazine for a period of at least 6 weeks, each without significant symptomatic relief (Kane et al. 1988a). None of the patients had ever been treated with atypical neuroleptics. Patients underwent a washout period of 2–4 weeks. The use of treatment refractory patients who were on relatively large doses of typical neuroleptics, as well as the neuroleptic washout period, is advantageous, because these conditions provide each patient with the opportunity to more fully express the TD phenotype, considering that they may be carrying the genetic predisposition for this disorder. The masking of the TD phenotype is a frequent occurrence and is represented in the subtypes of TD described in Schooler and Kane (1982). Following the neuroleptic washout, patients were assessed for TD severity using either the Abnormal Involuntary Movement Scale (Guy 1976) or a modified Hillside Simpson Dyskinesia scale, which was used for the 25 patients obtained from Hillside Hospital. The seven body area items and the over-all global item of the modified Hillside Simpson Dyskinesia scale duplicate the items of the AIMS. Thus, we were able to extract AIMS scores for each of the 25 patients that had been assessed using the modified Hillside Simpson Dyskinesia scale. All three clinicians (HYM, JAL, SGP) have extensive experience in assessing TD severity and exchange visits to all three of the clinical sites were arranged to enhance the consistency of the AIMS methodology.
Genotyping
Blood samples were collected from each of the clinical sites and were analyzed at the Clarke Institute of Psychiatry, Toronto. Genomic DNA was extracted from whole blood using a nonenzymatic, high-salt procedure (Lahiri and Nurnberger 1991). The polymerase chain reaction (PCR) was performed in a Perkin–Elmer Cetus Thermal Cycler (model 9600) to amplify the desired polymorphic portion of the D3 receptor gene. A single nucleotide substitution exists in the first exon of the D3 receptor gene, which results in a serine to glycine change in the amino acid sequence of the receptor protein. A 462 bp segment containing this nucleotide substitution was amplified by PCR (Lannfelt et al. 1992). Amplification reactions were performed in a total volume of 25 ml, and each reaction contained 100 ng of genomic DNA, 0.16 mM of each dNTP (dATP, dTTP, dGTP, dCTP), 1X reaction buffer (Cetus; 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 0.001% gelatin), 1.5 mM MgCl2, 0.01% gelatin, 1 unit of Taq polymerase, and 10 picomoles of each primer. Primer sequences were : 5′-GCT CTA TCT CCA ACT CTC ACA-3′, and 5′-AAG TCT ACT CAC CTC CAG GTA-3′. Template genomic DNA was denatured at 99°C for 3 min before adding the reaction mixture. Then, 35 cycles were performed, each consisting of 95°C for 20 s, 56°C for 20 s, and 72°C for 20 s. PCR products were digested overnight with 1 unit of MscI restriction endonuclease. An internal control in the form of a second MscI site confirmed whether or not the digestion reaction was accomplished. The digested PCR products were then subjected to electrophoresis in a 3.5% agarose gel and visualized using ethidium bromide. Genotyping was performed as described elsewhere (Lannfelt et al. 1992).
Statistical Methods
Analysis of covariance (ANCOVA) was used to compare the mean AIMS scores for each of the genotypic classes. The ANCOVA statistic was chosen because of its ability to incorporate additional factors as well as covariates into the statistical model. Based on previously published data implicating increased age as a clinical risk factor for TD (Johnson et al. 1982; Kane et al. 1988b; Miller and Jankovic 1990; Smith and Baldessarini 1980), age was used as a covariate in the ANCOVA. The demographics of our sample were statistically analyzed to determine any effects of sex and ethnicity that may have confounded the ANCOVA statistic. The effects of sex and ethnicity were tested using a one-way analysis of variance (ANOVA), and the effect of age was tested using linear regression analysis. Given that several studies have implicated female gender and African American ancestry as clinical risk factors for TD (Eastham et al. 1996; Jeste et al. 1996; Kane 1992; Kane and Smith 1982; Morgenstern and Glazer 1993; Yassa et al. 1983), both sex and ethnicity were included as factors in the modified ANCOVA model. The assumptions of the ANCOVA statistic were tested to determine the appropriateness of our sample. The assumption of equal variance between each genotypic class was tested using the Levene Test for Homogeneity of Variances. Chi-square analysis was used to test whether the distribution of genotypes was in accordance with Hardy–Weinberg equilibrium. Power calculations were performed based on mean AIMS scores and sample sizes, with alpha set to 0.05. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS), version 7.0.
RESULTS
No statistically significant differences were found in the distribution of sex or ethnicity among the samples from the three different clinical sites. There was a predominance of Caucasian and male patients in all three clinical samples (Table 1). However, the proportion of male to female patients and the proportion of Caucasian to other patients were similar in all three clinical sites. There was a significant difference observed between the mean age of the patients across the three clinical sites (F[2,107] = 8.31, p = .0004) and using a Student–Newman-Keuls test, the mean ages of the Meltzer and Lieberman samples were significantly lower than those of the Potkin sample (Table 1). The samples were combined into one group, producing a total of 112 patients. Despite previously published data implicating female gender as a risk factor for TD (Kane 1992; Yassa and Jeste 1992), in this combined sample, no statistically significant difference was found between the sexes regarding TD severity (F[1,108] = 0.89, p = .347). Other studies have also reported similar TD incidence rates in both males and females (Jeste et al. 1996; Waddington et al. 1995). In accordance with previously published clinical data (Eastham et al. 1996; Jeste et al. 1996; Kane 1992; Kane and Smith 1982; Morgenstern and Glazer 1993; Yassa et al. 1983), it was found that the African Americans in the sample had higher mean AIMS scores (mean = 10.68 ± 12.19 SD) than the other patients of different ethnic origins (Caucasian mean = 4.73 ± 6.59 SD; Asian mean = 5.42 ± 8.04 SD) (F[2,109] = 5.12, p = .0075). Also in agreement with previously published clinical data (Johnson et al. 1982; Kane et al. 1988b; Miller and Jankovic 1990; Smith and Baldessarini 1980), a significant linear relationship was found between age and TD severity. More specifically, as age increased, patients were more likely to exhibit higher AIMS scores (Pearson r = 0.25, p = .017).
Genotype frequencies for the DRD3 MscI polymorphism did not differ significantly among patients from different clinical sites or between males and females. Also, mean age did not differ significantly between the patients grouped according to genotype (F[2,109] = 0.13, p = .88). However, there was a statistically significant difference between genotype frequency and ethnic status (χ2 = 44.55, df = 2, p < .000005). African Americans had a significantly higher occurrence of the glycine allele of the Ser9Gly polymorphism than did the Caucasians. When testing for Hardy–Weinberg equilibrium between the alleles of this polymorphism, it was found that genotype frequencies in this sample did not deviate significantly from the frequencies expected under random mating conditions (χ2 = 0.13, df = 2, p = .939). Also, the allele frequencies in this sample were similar to those previously reported by other groups.
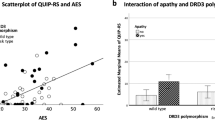
In light of the above demographic findings, a modified ANCOVA model was designed that corrects for the effects of sex and ethnicity. Age was used as a covariate in the analysis, and all the assumptions of the ANCOVA; namely, normally distributed genotypic categories with equal variances across all groups were evaluated to ensure the validity of the test. It was found that the serine to glycine polymorphism in the DRD3 receptor was significantly associated with typical neuroleptic-induced TD (F[2,95] = 8.25, p < .0005), with a statistical power of 0.568 and an r-square value of 0.297. Figure 1 is a graphic representation of this finding. A post hoc Student–Newman-Keuls test revealed higher mean AIMS scores in patients homozygous for the glycine allele of the DRD3 gene (mean AIMS of 14.20 ± 12.49 SD) when compared to heterozygous patients (mean AIMS of 3.92 ± 5.33 SD) and patients homozygous for the serine allele (mean AIMS of 3.47 ± 4.34 SD). To test for effects of ethnic stratification, we conducted an analysis that separated Caucasians and African Americans. It was found that in both Caucasians (n = 85, F[2,75] = 3.85, p = .026) and African Americans (n = 25, F[1,23] = 8.10, p = .0091), patients homozygous for the glycine allele were more likely to exhibit higher AIMS scores (Figure 2 ).
Mean corrected AIMS scores for each of the genotypic classes. The variances(s2) for each of the genotypic classes were as follows: (s2 for Ser/Ser genotype was 18.8; s2 for Ser/Gly genotype was 28.4; and s2 for Gly/Gly genotype was 156.1). The Levene Test for homogeneity of variances revealed a significant difference in the variances among the genotypic classes, thus violating one of the assumptions of the ANCOVA model. However, a nonparametric alternative, the Kruskal–Wallis Test, illustrated similar results (χ2 = 13.6644, df = 2, p = .0011, p = 0.0033 Bonferroni corrected).
Ethnically stratified analysis. To address the issue of population stratification, an analysis separating Caucasians from African Americans was conducted. It was found that in both ethnic samples, patients with Gly/Gly genotypes were more likely to exhibit larger AIMS scores, thus reducing the possibility of a false positive attributable to ethnic stratification. Figure 2A illustrates the Caucasian results, and Figure 2B gives the African American results.
The data values within each of the genotypic groups were normally distributed; however, the Levene Test for homogeneity of variances revealed that the variances for each genotypic class were not equal (Levene statistic = 42.97, df1 = 2, df2 = 111, p < .0005), and as such, one of the assumptions of the ANCOVA model had been violated (Figure 1). In light of this, a less powerful nonparametric alternative statistic, the Kruskal–Wallis H test, was applied to test the specific hypothesis of DRD3 involvement in TD. Using this statistic, the same association was found between homozygosity for the glycine allele and increased mean AIMS score (the SPSS software package uses the χ2 distribution as an approximation of the Kruskal–Wallis H distribution; χ2 = 13.66, df = 2, p = .0011).
DISCUSSION
We have observed a statistically significant association between the glycine variant of DRD3 and TD. AIMS scale scores were utilized to assess TD severity in 112 treatment refractory schizophrenia patients. It was found that mean AIMS scores for individuals homozygous for the glycine variant were significantly higher than individuals who were either serine/serine homozygotes or serine/glycine heterozygotes. This study was unique as compared to other studies, in that it took into consideration several demographic variables that are known to influence the severity of TD and, to our knowledge, is the first study to use a more powerful parametric statistic (ANCOVA). The ANCOVA method of analysis chosen is more informative than traditional case-control χ2/Fisher's Exact tests that studies to date have used. By utilizing continuous AIMS scale scores as the dependent variable in the ANCOVA, we do not lose valuable information regarding the severity of the phenotype. The published case-control studies of TD are subject to this problem, because they use TD diagnostic criteria to subdivide the sample into those with TD and those without. For example, in such studies, patients with AIMS scores of 10 are categorized as having TD and are indistinguishable from patients with AIMS scores of 40 who are indiscriminately grouped into this category. From a statistical perspective, the ANCOVA F statistic does not require as large a sample size to detect a significant association, and as such, is more powerful. Furthermore, this statistic allows for the direct incorporation of demographic variables that also affect the phenotype, and thus directly controls for these potential confounding factors.
It is possible that the substitution of a polar serine residue to a nonpolar glycine residue may alter the tertiary structure of the D3 receptor. A recent report by Lundstrom and Turpin (1996) has identified functional differences in dopamine affinity for each of these receptor alleles as expressed in CHO cell lines. They reported significantly higher dopamine affinity for the glycine homozygote cells, as compared to both heterozygote binding and serine/serine homozygotes. It is interesting to note that patients homozygous for the glycine variant display more severe TD and that the aforementioned study of Lundstrom and Turpin found increased dopamine affinity in CHO cell lines homozygous for the glycine variant. Perhaps these functional differences may contribute to the TD phenotype, although it is difficult to speculate further on the precise mechanism involved. Thus, this could suggest that the higher affinity of the glycine allele may be involved in the pathogenesis of TD. Alternatively, this Ser9Gly polymorphism may be in linkage disequilibrium with another site conferring susceptibility to TD. Asherson and colleagues (Asherson et al. 1996) screened all six exons of DRD3 in 72 DNA samples using SSCP and did not find any additional coding variants, hence DRD3 may be in linkage disequilibrium with another neighboring locus. Although the possibility of a false positive finding cannot be ruled out.
It is important to note that the r-squared value for our result was 0.297, thus indicating that approximately 30% of the variance observed in the TD phenotype can be accounted for by the proposed ANCOVA model. Of the various factors examined in this model (D3 genotype, age, sex, and ethnicity), D3 genotype was the only factor that significantly contributed to the TD phenotype within the over-all ANCOVA model. The relatively low r-squared value suggests that other factors, possibly genetic variation in other genes, as well as additional environmental influences, may account for the remaining 70% of the variance in TD phenotype.
To justify merging the patients from each clinical site into one single sample, data from each of the different sites were compared to ensure that each was a proper representation of a random sample. Although no differences were found among the different sites regarding sex and ethnic distributions, mean age was found to be slightly higher in the Potkin sample (Table 1), likely because of the relatively small sample size (n = 13). This difference in age was not considered a sufficient reason to eliminate the sample from the study.
Epidemiological data suggest that the occurrence of TD is likely to be highly related to a genetic predisposition, the effect of which may become expressed in conjunction with other variables, in particular age, ethnicity, sex, and neuroleptic history (Cavallaro and Smeraldi 1995). Where possible, demographic variables were assessed in our sample to determine any external effects that may have confounded the ANCOVA analysis; therefore, age, sex, and ethnicity were included as contributing factors in the analysis. In accordance with previously published data, our sample shows that increased age and African American descent are clinical risk factors for TD. Our sample included a wide age range (16–58 years), which may have led to a sampling bias. For example, if by chance alone, we sampled a greater number of older individuals homozygous for the glycine variant and very few young individuals of the same genotype, then given increased age as a risk factor for TD, a false-positive association may have occurred for DRD3 and TD. However, this was not the case in our sample because there were no differences between the mean ages of patients in the three genotypic classes studied (p = .88).
As evidenced by this study, it is important to account for the putative effects of both clinical and demographic, components of the sample, as these may confound genetic studies. For example, African Americans were found to have a significantly higher occurrence of the glycine allele; furthermore, the African Americans in our sample had higher mean AIMS scores. Therefore, it is quite possible that a population stratification bias could have been responsible for the positive association we are reporting. In order to address this crucial issue, an additional analysis that divided the sample according to ethnicity was conducted between the Caucasians and African Americans (Figure 2). Given that the association between DRD3 genotype and TD remains positive in the Caucasian sample (F[2,75] = 3.85, p = .026) as well as in the African American sample (F[1,23] = 8.10, p = .0091), it is unlikely that an ethnic stratification bias has led to our positive result.
An important limitation of this study is that total neuroleptic histories for all patients were unavailable and, thus, were not incorporated into the ANCOVA model. This variable is important as a clinical risk factor for TD and should be controlled for in genetic studies of TD (Jeste and Kelsoe 1997). However, all our patients met the criteria for treatment-refractory or intolerant disease (Kane et al. 1988a), and were treated with typical neuroleptics for at least 1 year before being recruited for the study.
Another limitation was the use of two different assessement scales for TD severity; namely, the AIMS scale in the 87 patients from both the Meltzer and Potkin samples, and the Hillside Simpson Dyskinesia scale in the 25 patients from the Lieberman sample. Although many of the items in each are very similar and address the same issues, the different scales may have different sensitivities, and the conversion of one scale to the other may introduce sources of error. Although this discrepancy is a limitation of the study, it is unlikely that the results are biased because of this. When eliminating all 25 patients with Hillside Simpson Dyskinesia scores, the remaining 87 patients with AIMS scores likewise produce a significant association between DRD3 genotype and TD severity (F[2,73] = 4.40, p = .016). Currently, there are several different rating scales used to assess TD severity; however, there has been no general consensus reached as to which scale best measures TD severity. A standard TD diagnostic instrument should be agreed upon for use in future studies.
A potential confounding factor in this study, as well as in most genetic studies, is the problem of phenotypic heterogeneity. On one level, several studies have reported sporadic cases of dyskinetic movements and various other dyskinesias in neuroleptic-naive psychiatric patients (Awouters et al. 1990; Cassady et al. 1998; Chatterjee et al. 1995; Kopala 1996). This study did not take this factor into account because it was indeterminable whether patients exhibited TD because of neuroleptic treatment or not. It is quite possible that a subset of our patient sample exhibited spontaneous dyskinesias that may have different underlying pathophysiological mechanisms from those responsible for neuroleptic-induced TD. This may explain our modest r-squared value of 0.297. On another level, it is possible that the TD phenotype may be heterogeneous regarding the localization of the abnormal involuntary movement. Some patients exhibit only orofacial TD; whereas, others demonstrate abnormal involuntary movements of their extremities and truncal regions. A recent preliminary report by Macciardi and colleagues (1996) found a positive association between this Ser9Gly polymorphism of DRD3 and orofacial TD symptomatology; dyskinetic movements of the trunk and extremities were not associated with this polymorphism. Hence, it is plausible that TD exhibited in these various body regions may have different underlying pathophysiological mechanisms. Although data were available allowing for the stratification of patients with regard to body regions afflicted, our sample, when broken down into these clinical subgroups, did not exhibit enough statistical power to allow for this stratified analysis. At the present time, it remains unclear as to whether or not the clinical subtypes of TD (spontaneous dyskinesias, body localization, probable TD, masked TD, transient TD, withdrawal TD, persistent TD, masked persistent TD) have distinct underlying etiologies. Until larger sample sizes are available for each of these subtypes, this issue of heterogeneity will remain unclear. Also, given that our study examined patients with schizophrenia, it is quite possible that our sample is heterogeneously biased toward a clinical subtype of schizophrenia that is more prone to result in TD. For example, the underlying true association may be between DRD3 and a more severe and/or treatment refractory form of schizophrenia that requires a higher neuroleptic dose for treatment, and, as such, these patients are more likely to exhibit TD.
A statistical limitation of this study was that the ANCOVA assumption of equal variances across genotypic groups was violated. As a result, we employed a less powerful, nonparametric Kruskal–Wallis H test, which is unaffected by violation of this assumption. The result of this test was statistically significant, suggesting over-all association of this DRD3 polymorphism with TD. However, this nonparametric test does not allow for the incorporation of additional factors, hence demographic variables could not be considered in the model. Given that DRD3 genotype was the only significant factor in the parametric ANCOVA model and that the nonparametric test produced a p value of .0011, it is unlikely that this limitation of the Kruskal–Wallis H test is of major concern.
Another statistical problem with this study is the issue of multiple testing. In addition to the main ANCOVA model, we performed a Kruskal–Wallis H test, as well as an analysis separating Caucasians and African Americans. We account for this by presenting Bonferroni corrected p values. As with all genetic association studies, replication of the over-all result in an independent sample is required to confirm our finding. This sample is currently in the process of being collected.
The results presented in this study suggest the involvement of the Ser9Gly DRD3 polymorphism as a predisposing risk factor for TD. This genetic information may, in future, assist clinicians in determining patient susceptibility to TD and ultimately may aid in the elucidation of the pathophysiological mechanisms.
References
Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, Gerfen CR, Westphal H, Fuchs S . (1996): A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Nat Acad Sci USA 93: 1945–1949
APA. (1994): Diagnostic and Statistical Manual of Mental Disorders, 4th Ed, (DSM-IV). Washington, DC, American Psychiatric Association (APA)
Asherson P, Mant R, Holmans P, Williams J, Cardno A, Murphy K, Jones L, Collier D, McGuffin P, Owen MJ . (1996): Linkage, association, and mutational analysis of the dopamine D3 receptor gene in schizophrenia. Mol Psychiat 1: 125–132
Awouters F, Niemegeers CJ, Janssen PA . (1990): “Tardive” dyskinesia: Etiological and therapeutic aspects. Pharmacopsychiatry 23: 33–37
Badri F, Masellis M, Petronis A, Macciardi FM, Van Tol HHM, Cola P, Meltzer HY, Lieberman J, Potkin S, Kennedy JL . (1996): Dopamine and serotonin system genes may predict clinical response to clozapine. Proceedings of the 46th Annual Meeting of the American Society of Human Genetics, vol 59. San Francisco, American Journal of Human Genetics, p A247
Buchanan RW . (1995): Clozapine: Efficacy and safety. Schizophr Bull 21: 579–591
Buckland PR, McGuffin P . (1992): Changes in dopamine D1, D2, and D3 receptor mRNA levels in rat brain following antipsychotic treatment. Psychopharmacology 106: 479–483
Caligiuri MP, Harris MJ, Jeste DV . (1988): Quantitative analyses of voluntary orofacial motor control in schizophrenia and tardive dyskinesia. Biol Psychiat 24: 787–800
Casey DE . (1991): Neuroleptic drug-induced extrapyramidal syndromes and tardive dyskinesia. Schizophr Res 4: 109–120
Casey DE . (1992): Dopamine D1 (SCH 23390) and D2 (haloperidol) antagonists in drug-naive monkeys. Psychopharmacology 107: 18–22
Casey DE, Gerlach J, Bjorndal N . (1982): Levodopa and receptor sensitivity modification in tardive dyskinesia. Psychopharmacology 78: 89–92
Cassady SL, Adami H, Moran M, Kunkel R, Thaker GK . (1998): Spontaneous dyskinesia in subjects with schizophrenia spectrum personality. Am J Psychiat 155: 70–75
Cavallaro R, Smeraldi E . (1995): Recognition, avoidance, and management of antipsychotic-induced tardive dyskinesia. CNS Drugs 4: 278–293
Chatterjee A, Chakos M, Koreen A, Geisler S, Sheitman B, Woerner M, Kane JM, Alvir J, Lieberman JA . (1995): Prevalence and clinical correlates of extrapyramidal signs and spontaneous dyskinesia in never-medicated schizophrenic patients. Am J Psychiat 152: 1724–1729
Chen CH, Wei FC, Koong FJ, Hsiao KJ . (1997): Association of TaqI a polymorphism of dopamine D-2 receptor gene and tardive dyskinesia in schizophrenia. Biolog Psychiat 41: 827–829
Eastham JH, Lacro JP, Jeste DV . (1996): Ethnicity and movement disorders. Mt Sinai J Med 63: 314–319
Fink-Jensen A, Nielsen EB, Hansen L, Scheideler MA . (1998): Behavioral and neurochemical effects of the preferential dopamine d3 receptor agonist cis-8-OH-PBZI. Eur J Pharmacol 342: 153–161
Gaitonde EJ, Morris A, Sivagnanasundaram S, McKenna PJ, Hunt DM, Mollon JD . (1996): Assessment of association of D3 dopamine receptor MscI polymorphism with schizophrenia: Analysis of symptom ratings, family history, age at onset, and movement disorders. Am J Med Genet 67: 455–458
Gendreau PL, Petitto JM, Schnauss R, Frantz KJ, Van Hartesveldt C, Gariepy JL, Lewis MH . (1997): Effects of the putative dopamine D3 receptor antagonist PNU 99194A on motor behavior and emotional reactivity in C57BL/6J mice. Eur J Pharmacol 337: 147–155
Gerlach J, Casey DE . (1984): Sulpiride in tardive dyskinesia. Acta Psychiat Scand 311: 93–102
Gordon JH, Fields JZ . (1989): A permanent dopamine receptor up-regulation in the ovariectomized rat. Pharmacol Biochem Behav 33: 123–125
Guala A, Mittino D, Ghini T, Quazza G . (1992): Are metoclopramide dystonias familial? Pediatr Med Chir 14: 617–618
Gurevich EV, Bordelon Y, Shapiro RM, Arnold SE, Gur RE, Joyce JN . (1997): Mesolimbic dopamine D3 receptors and use of antipsychotics in patients with schizophrenia. A postmortem study. Arch Gen Psychiat 54: 225–232
Guy W, ed (1976): Early Clinical Drug Evaluation Unit Assessment Manual. Rockville Maryland, U.S. Department of Health and Human Services, National Institute of Mental Health (NIMH).
Hashimoto T, Ross DE, Gao XM, Medoff DR, Tamminga CA . (1998): Mixture in the distribution of haloperidol-induced oral dyskinesias in the rat supports an animal model of tardive dyskinesia. Psychopharmacology (Berl) 137: 107–112
Hoffman WF, Labs SM, Casey DE . (1987): Neuroleptic-induced parkinsonism in older schizophrenics. Biol Psychiat 22: 427–439
Inada T, Dobashi I, Sugita T, Inagaki A, Kitao Y, Matsuda G, Kato S, Takano T, Yagi G, Asai M . (1997): Search for a susceptibility locus to tardive dyskinesia. Human Psychopharmacol Clin Exper 12: 35–39
Jeste DV, Caligiuri MP . (1993): Tardive dyskinesia. Schizophr Bull 19: 303–315
Jeste DV, Kelsoe JR . (1997): Schizophrenia, tardive dyskinesia, and a D3 receptor gene variant: A new twist on dyskinesias? Molec Psychiat 2: 86–88
Jeste DV, Lindamer LA, Evans J, Lacro JP . (1996): Relationship of ethnicity and gender to schizophrenia and pharmacology of neuroleptics. Psychopharmacol Bull 32: 243–251
Johnson GF, Hunt GE, Rey JM . (1982): Incidence and severity of tardive dyskinesia increase with age [letter]. Arch Gen Psychiat 39: 486
Joyce JN, Meador-Woodruff JH . (1997): Linking the family of D-2 receptors to neuronal circuits in human brain: Insights into schizophrenia. Neuropsychopharmacology 16: 375–384
Kane JM . (1992): Clinical efficacy of clozapine in treatment-refractory schizophrenia: An overview. Brit J Psychiat 160: 41–45
Kane JM, Honigfeld G, Singer J, Meltzer H, Group CCS . (1988a): Clozapine for the treatment-resistant schizophrenic: A double-blind comparison with chlorpromazine. Arch Gen Psychiat 45: 789–796
Kane JM, Smith JM . (1982): Tardive dyskinesia: Prevalence and risk factors, 1959 to 1979. Arch Gen Psychiat 39: 473–481
Kane JM, Woerner M, Lieberman J . (1988b): Tardive dyskinesia: Prevalence, incidence, and risk factors. J Clin Psychopharmacol 8: 52S–56S
Kane JM, Woerner MG, Pollack S, Safferman AZ, Lieberman JA . (1993): Does clozapine cause tardive dyskinesia? J Clin Psychiat 54: 327–330
Kling-Petersen T, Ljung E, Svensson K . (1995): Effects on locomotor activity after local application of D3 preferring compounds in discrete areas of the rat brain. J Neural Transm 102: 209–220
Kopala LC . (1996): Spontaneous and drug-induced movement disorders in schizophrenia. Acta Psychiat Scand 389: 12–17
Lahiri DK, Nurnberger JI, Jr . (1991): A rapid nonenzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res 19: 5444
Lannfelt L, Sokoloff P, Martres MP, Pilon C, Giros B, Jonsson E, Sedvall G, Schwartz JC . (1992): Amino acid substitution in the dopamine D3 receptor as a useful polymorphism for investigating psychiatric disorders. Psychiat Genet 2: 249–256
Lieberman J, Pollack S, Lesser M, Kane J . (1988): Pharmacologic characterization of tardive dyskinesia. J Clin Psychopharmacol 8: 254–260
Lieberman JA, Saltz BL, Johns CA, Pollack S, Borenstein M, Kane J . (1991): The effects of clozapine on tardive dyskinesia. Br J Psychiat 158: 503–510
Lundstrom K, Turpin MP . (1996): Proposed schizophrenia-related gene polymorphism: Expression of the Ser9Gly mutant human dopamine D3 receptor with the Semliki Forest virus system. Biochem Biophys Res Commun 225: 1068–1072
Macciardi F, Verga M, Cavallaro R, Pedrini S, Cohen S, Smeraldi E . (1996): A genetic study of tardive dyskinesia in an Italian population of chronic schizophrenics. Proceedings of World Congress on Psychiatric Genetics, San Francisco, Psychiatric Genetics
Meador-Woodruff JH, Haroutunian V, Powchik P, Davidson M, Davis KL, Watson SJ . (1997): Dopamine receptor transcript expression in striatum and prefrontal and occipital cortex. Arch Gen Psychiat 54: 1089–1095
Meshul CK, Casey DE . (1989): Regional, reversible ultrastructural changes in rat brain with chronic neuroleptic treatment. Brain Res 489: 338–346
Miller LG, Jankovic J . (1990): Neurologic approach to drug-induced movement disorders: A study of 125 patients. South Med J 83: 525–532
Morgenstern H, Glazer WM . (1993): Identifying risk factors for tardive dyskinesia among long-term outpatients maintained with neuroleptic medications. Results of the Yale Tardive Dyskinesia Study. Arch Gen Psychiat 50: 723–733
Muller DJ, Ahle G, Alfter D, Krauss H, Knapp M, Marwinski K, Schulze TG, Weber T, Nothen M, Maier W, Held T, Rietschel M . (1998): Familial occurrence of tardive dyskinesia. Proceedings of the 6th World Congress on Psychiatric Genetics, Bonn, Germany. Am J Med Genetics 81: 527
O'Callaghan E, Larkin C, Kinsella A, Waddington JL . (1990): Obstetric complications, the putative familial-sporadic distinction, and tardive dyskinesia in schizophrenia. Br J Psychiat 157: 578–584.
Ranjan R, Meltzer HY . (1996): Acute and long-term effectiveness of clozapine in treatment-resistant psychotic depression. Biol Psychiat 40: 253–258
Rosengarten H, Schweitzer JW, Friedhoff AJ . (1993a): A mechanism underlying neuroleptic-induced oral dyskinesias in rats. Pol J Pharmacol 45: 391–398
Rosengarten H, Schweitzer JW, Friedhoff AJ . (1993b): A subpopulation of dopamine D1 receptors mediate repetitive jaw movements in rats. Pharmacol Biochem Behav 45: 921–924
Rosengarten H, Schweitzer JW, Friedhoff AJ . (1994): Possible genetic factors underlying the pathophysiology of tardive dyskinesia. Pharmacol Biochem Behav 49: 663–667
Sasaki T, Kennedy JL, Nobrega JN . (1996): Autoradiographic mapping of mu opioid receptor changes in rat brain after long-term haloperidol treatment: Relationship to the development of vacuous chewing movements. Psychopharmacology (Berl) 128: 97–104.
Sasaki T, Kennedy JL, Nobrega JN . (1997): Localized changes in GABA receptor-gated chloride channel in rat brain after long-term haloperidol: Relation to vacuous chewing movements. Synapse 25: 73–79
Schooler NR, Kane JM . (1982): Research diagnoses for tardive dyskinesia. Arch Gen Psychiat 39: 486–487
Smith JM, Baldessarini RJ . (1980): Changes in prevalence, severity, and recovery in tardive dyskinesia with age. Arch Gen Psychiat 37: 1368–1373
Steen VM, Lovlie R, MacEwan T, McCreadie RG . (1997): Dopamine D3-receptor gene variant and susceptibility to tardive dyskinesia in schizophrenic patients. Molecular Psychiat 2: 139–145
Suzuki M, Hurd YL, Sokoloff P, Schwartz JC, Sedvall G . (1998): D3 dopamine receptor mRNA is widely expressed in the human brain. Brain Res 779: 58–74
Swartz JR, Burgoyne K, Smith M, Gadasally R, Ananth J, Ananth K . (1997): Tardive dyskinesia and ethnicity: Review of the literature. Ann Clin Psychiat 9: 53–59
Tarsy D, Baldessarini RJ . (1977): The pathophysiologic basis of tardive dyskinesia. Biol Psychiat 12: 431–450
Van Hartesveldt C . (1997): Temporal and environmental effects on quinpirole-induced biphasic locomotion in rats. Pharmacol Biochem Behav 58: 955–960
Waddington JL, O'Callaghan E, Buckley P, Madigan C, Redmond O, Stack JP, Kinsella A, Larkin C, Ennis JT . (1995): Tardive dyskinesia in schizophrenia. Relationship to minor physical anomalies, frontal lobe dysfunction, and cerebral structure on magnetic resonance imaging. Br J Psychiat 167: 41–44
Waddington JL, Youssef HA . (1988): The expression of schizophrenia, affective disorder, and vulnerability to tardive dyskinesia in an extensive pedigree. Br J Psychiat 153: 376–381
Weinhold P, Wegner JT, Kane JM . (1981): Familial occurrence of tardive dyskinesia. J Clin Psychiat 42: 165–166
Wilson WH . (1992): Clinical review of clozapine treatment in a state hospital. Hosp Comm Psychiat 43: 700–703
Wolfe ME, Mosnaim AD . (1988): Tardive Dyskinesia: Biological Mechanisms and Clinical Aspects. Washington, DC, American Psychiatric Press
Yassa R, Ananth J . (1981): Familial tardive dyskinesia. Am J Psychiat 138: 1618–1619
Yassa R, Ananth J, Cordozo S, Ally J . (1983): Tardive dyskinesia in an outpatient population: Prevalence and predisposing factors. Can J Psychiat 28: 391–394
Yassa R, Jeste DV . (1992): Gender differences in tardive dyskinesia: A critical review of the literature. Schizophr Bull 18: 701–715
Youssef H, Lyster G, Youssef F . (1989): Familial psychosis and vulnerability to tardive dyskinesia. Int Clin Psychopharmacol 4: 323–328
Acknowledgements
JLK is the recipient of a NARSAD Independent Investigator award. This work was supported by the Medical Research Council of Canada and the Ontario Mental Health Foundation.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Basile, V., Masellis, M., Badri, F. et al. Association of the MscI Polymorphism of the Dopamine D3 Receptor Gene with Tardive Dyskinesia in Schizophrenia. Neuropsychopharmacol 21, 17–27 (1999). https://doi.org/10.1016/S0893-133X(98)00114-6
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(98)00114-6
Keywords
This article is cited by
-
A preclinical secondary pharmacology resource illuminates target-adverse drug reaction associations of marketed drugs
Nature Communications (2023)
-
Antipsychotic-induced vacuous chewing movements and extrapyramidal side effects are highly heritable in mice
The Pharmacogenomics Journal (2012)
-
Association study of Cannabinoid receptor 1 (CNR1) gene in tardive dyskinesia
The Pharmacogenomics Journal (2012)
-
Genetics of Antipsychotic-induced Side Effects and Agranulocytosis
Current Psychiatry Reports (2011)
-
Association study of tardive dyskinesia and five DRD4 polymorphisms in schizophrenia patients
The Pharmacogenomics Journal (2009)