Abstract
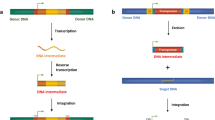
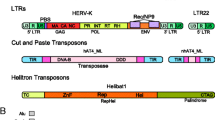
Transposable elements are short but complex pieces of DNA or RNA containing a streamlined minimal-genome with the capacity for its selfish replication in a foreign genomic environment. Cis-regulatory sections within the elements orchestrate tempo and mode of TE expression. Proteins encoded by TEs mainly direct their own propagation within the genome by recruitment of host-encoded factors. On the other hand, TE-encoded proteins harbor a very attractive repertoire of functional abilities for a cell. These proteins mediate excision, replication and integration of defined DNA fragments. Furthermore, some of these proteins are able to manipulate important host factors by altering their original function. Thus, if the host genome succeeds in domesticating such TE-encoded proteins by taming their ‘anarchistic behavior,’ such an event can be considered as an important evolutionary innovation for its own benefit. In fact, the domestication of TE-derived cis-regulatory modules and protein coding sections took place repeatedly in the course of genome evolution. We will present prominent cases that impressively demonstrate the beneficial impact of TEs on host biology over evolutionary time. Furthermore, we will propose that molecular domestication might be considered as a resumption of the same evolutionary process that drove the transition from ‘primitive genomes’ to ‘modern’ ones at the early dawn of life, that is, the adaptive integration of a short piece of autonomous DNA into a complex regulatory network.
Similar content being viewed by others
References
Agrawal A., Q.M. Eastman & D.G. Schatz, 1998. Transposition mediated by RAG1 and RAG2 and its implications for the evolution of the immune system. Nature 394: 744–751.
Andrews, J.D. & G.B. Gloor, 1995. A role for the KP leucine zipper in regulating P element transposition in Drosophila. Genetics 141: 587–594.
Berg, D.E. & M.M. Howe, 1989. Mobile DNA, Am. Soc. Microbiol., Washington, DC.
Best, S., P. Le Tissier, G. Towers & J.P. Stoye, 1996. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature 382: 826–829.
Biessmann, H., A. Valgiersdottir, A. Lofsky, C. Chin, B. Ginther, R. Levis & M.P. Pardue, 1992. Het-A, a transposable element specifically involved in ‘healing’ broken chromosome ends in Drosophila. Mol. Cell. Biol. 12: 3910–3918.
Boeke. J.D., 1997. LINEs and Alus — the polyA connection. Nat. Genet. 16: 6–7.
Britten, R., 1996. DNA sequence insertion and evolutionary variation in gene regulation. Proc. Natl. Acad. USA 93: 9374–9377.
Brosius, J., 1999. RNAs from all categories generate retrosequences that may be exapted as novel genes or regulatory elements. Gene 238: 115–134.
Clark, J.B. & M.G. Kidwell, 1997. A phylogenetic perspective of P transposable element evolution in Drosophila. Proc. Natl. Acad. Sci. USA 94: 11428–11433.
Charlesworth, B., P. Sniegowski & W. Stephan, 1994. The evolutionary dynamics of repetitive DNA in eukaryotes. Nature 371: 215–220.
Cordonnier A., J.F. Casella & T. Heidmann, 1995. Isolation of novel human endogenous retrovirus-like elements with foamy virus-related pol sequence. J. Virol. 69: 5890–5897.
Danilevskaya, O., A. Lofsky, E. Kurenova & M.L. Pardue, 1993. The Y chromosome of Drosophila melanogaster contains a distinctive subclass of Het-A-related repeats. Genetics 134: 531–543.
Doolittle, W.F. & C. Sapienza, 1980. Selfish genes, the phenotype paradigm and genome evolution. Nature 284: 601–603.
Dorer, D. & S. Henikoff, 1994. Expansions of transgene repeats cause heterochromatin formation and gene silencing in Drosophila. Cell 77: 993–1002.
Dorer, D. & S. Henikoff, 1997. Transgene repeat arrays interact with distal heterochromatin and cause silencing in cis and trans. Genetics 147: 1181–1190.
Eickbush T., 1999. Telomerase and retrotransposons: which came first? Science 277: 911–912.
Engels, W.R., 1989. P-elements in Drosophila melanogaster, pp. 437–484, in Mobile DNA, edited by D.E. Berg, and M.M. Howe. American Society for Microbiology, Washington.
Gloor, G.B., C.R. Preston, D.M. Johnson-Schlitz, N.A. Nassif, R.W. Phillis, W.K. Benz, H.M. Robertson & W.R. Engels, 1993. Type I repressors of P element mobility. Genetics 135: 81–95.
Hagemann, S., W.J. Miller & W. Pinsker, 1992. Identification of a complete P element in the genome of Drosophila bifasciata. Nucl. Acids Res. 20: 409–413.
Hagemann, S., W.J. Miller & W. Pinsker, 1994. Two distinct P element subfamilies in the genome of Drosophila bifasciata. Mol. Gen. Genet. 244: 168–175.
Hagemann S., E. Haring & W. Pinsker, 1996. A new P element subfamily from Drosophila tristis, D. ambigua and D. obscura. Genome 39: 978–985.
Hagemann, S., W.J. Miller, E. Haring & W. Pinsker, 1998a. Nested insertions of short mobile sequences in Drosophila P elements. Chromosoma: 107: 6–16.
Hagemann, S., E. Haring & W. Pinsker, 1998b. Horizontal transmission vs. vertical inheritance of P elements in Drosophila and Scaptomyza: Has the M-type subfamily spread from East Asia? J. Zool. Syst. Evol. Res. 36: 75–83.
Haring, E., S. Hagemann, P. Lankinen & W. Pinsker, 1998. The phylogenetic position of Drosophila eskoi deduced from P element and Adh sequence data. Hereditas 128: 235–244.
Henikoff S. & M.A. Matzke, 1997. Exploring and explaining epigenetic effects. Trends Genet. 13: 293–295.
Hiom K., M. Melek & M. Gellert, 1998. DNA transposition by the RAG1 and RAG2 proteins: a possible source of oncogenic translocations. Cell 94: 463–470.
Jensen, S., M.P. Gassama & T. Heidmann, 1999. Taming of transposable elements by homolog-dependent gene silecing. Nat. Genet. 21: 209–212.
Jurka, J., 1998. Repeats in genomic DNA: mining and meaning. Curr. Opin. Struct. Biol. 8: 333–337.
Jurka, J. & V.V. Kapitonov, 1999. Sectorial mutagenesis by transposable elements. Genetica 107: 239–248.
Ketting R.F., T.H. Haverkamp, H.G. van Luenen & R.H. Plasterk 1999. Mut-7 of C. elegans, required for transposon silencing and RNA interference, is a homolog of Werner syndrome helicase and RNaseD. Cell 99: 133–141.
Kidwell, M.G., 1994. The evolutionary history of the P family of transposable elements. J. Hered. 85: 339–346.
Kidwell, M.G. & D. Lish, 1997. Transposable elements as source of variation in animals and plants. Proc. Natl. Acad. Sci. USA 94: 7704–7711.
Lee, C.C., Y.M. Mul & D.C. Rio, 1996. The Drosophila P-element KP repressor protein dimerizes and interacts with multiple sites on the P-element DNA. Mol. Cell. Biol. 16: 5616–5622.
Lee, C.C., E.L. Beall & D.C. Rio, 1998. DNA binding by the KP repressor protein inhibits P-element transposase activity in vitro. EMBO 17: 4166–4174.
Levis, R.W., R. Ganesan, K. Houtchens, L.A. Tolar & F. Sheen, 1993. Transposons in place of telomeric repeats at a Drosophila telomere. Cell, 75: 1083–1093.
Lingner J., T.R. Hughes, A. Shevchenko, M. Mann, V. Lundblad & T.R. Cech, 1997. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science 276: 561–567.
Long, Q., C. Bengra, C. Li, F. Kutlar & D. Tuan, 1998. A long terminal repeat of the human endogenous retrovirus ERV-9 is located in the 5′ boundary area of the human beta-globin locus control region. Genomics 54: 542–555.
Mason, J.M. & H. Biessmann, 1995. The unusual telomeres of Drosophila. Trends Genet. 11: 58–62.
McDonald, J.F., 1993. Evolution and consequences of transposable elements. Curr. Opin. Genet. Dev. 3: 855–864.
McDonald, J.F., 1995. Transposable elements: possible catalysts of organismic evolution. Trends Ecol. Evol. 10: 123–126.
McDonald J.F., 1998. Transposable elements, gene silencing and macroevolution. Trends Ecol. Evol. 13: 94–95.
Miller, W.J., S. Hagemann, E. Reiter & W. Pinsker 1992. P homologous sequences are tandemly repeated in the genome of Drosophila guanche. Proc. Natl. Acad. Sci. USA 89: 4018–4022.
Miller, W.J., N. Paricio, S. Hagemann, M.J. Martinez-Sebastián, W. Pinsker & R. DeFrutos, 1995. Structure and expression of the clustered P element homologues in Drosophila subobscura and D. guanche. Gene 156: 167–174.
Miller, W.J., L. Kruckenhauser & W. Pinsker, 1996. The impact of TEs on genome evolution in animals and plants, pp. 21–35 in Transgenic organisms: Risk assessment of deliberate release edited by K. Wöhrmann and J. Tomiuk. Birkhäuser, Basel.
Miller, W.J., J.F. McDonald & W. Pinsker, 1997. Molecular domestication of mobile elements. Genetica 100: 261–270.
Misra, S. & D.C. Rio, 1990. Cytotype control of Drosophila P element transposition: the 66 kd protein is a repressor of transposase activity. Cell 62: 269–284.
Misra, S., R.M. Buratowsky, T. Ohkawa & D.C. Rio, 1993. Cytotype control of Drosophila melanogaster P element transposition: genomic position determines maternal repression. Genetics 135: 785–800.
Nakayama J., M. Saito, H. Nakamura, A. Matsuura & F. Ishikawa, 1997. TLP1: a gene encoding a protein component of mammalian telomerase is a novel member of WD repeats family. Cell 88: 875–884.
Nouaud, D. & D. Anxolabéhère, 1997. P element domestication: a stationary P element may encode a 66 kDa repressor-like protein in the Drosophila montium species subgroup. Mol.Biol.Evol. 14: 1132–1144.
Nouaud, D., B. Boeda, L. Levy & D. Anxolabéhère, 1999. A P element has induced intron formation in Drosophila. Mol. Biol. Evol. 16: 1503–1510.
O'Hare, K. & G.M. Rubin, 1983. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell 34: 25–35.
O'Hare, K., A. Driver, S. McGrath & D.M. Johnson-Schlitz, 1992. Distribution and structure of cloned P elements from the Drosophila melanogaster P strain. Genet. Res. 60: 33–41.
Orgel, L.E. & F.H.C. Crick, 1980. Selfish DNA: the ultimate parasite. Nature 284: 604–607.
Pardue, M.L., O. Davilevskaya, K. Lowenhaupt, F. Slot & K.L Traverse, 1996. Drosophila telomeres: new views on chromosome evolution. Trends Genet. 12: 48–52.
Pardue, M.L., O.N. Danilevskaya, K.L. Traverse & K. Lowenhaupt, 1997. Evolutionary links between telomeres and transposable elements. Genetica 100: 73–84.
Paricio, N., M. Pérez-Alonso, M.J. Martínez-Sebastiá & R. de Frutos, 1991. P sequences of Drosophila subobscura lack exon 3 and may encode a 66 kd repressor-like protein. Nucl. Acids Res. 19: 6713–6718.
Paricio, N., W.J. Miller, W. Pinsker, S. Hagemann, R. deFrutos & M.J. Martinez-Sebastián, 1996. Structure and origin of the P element related gene cluster of Drosophila madeirensis. Genome 39: 823–829.
Pimpinelli S., M. Berloco, L. Fanti, P.S. Bonaccorsi, E. Marchetti, R. Caizzi, C. Caggese & M. Gatti, 1995. Transposable elements are stable structural components of the Drosophila melanogaster heterochromatin. Proc. Natl. Acad. Sci. USA 92: 3804–3808.
Pinsker, W., W.J. Miller & S. Hagemann, 1993. P-elements of Drosophila: Genomic parasites as genetic tools, pp. 25–42 in Transgenic organisms: Risk assessment of deliberate release, edited by K. Wöhrmann and J. Tomiuk. Birkhäuser, Basel.
Rio, D.C., 1990. Molecular mechanisms regulating Drosophila P element transposition. Annu. Rev. Genet. 24: 543–578.
Russo, C.A., N. Takezaki & M. Nei, 1995. Molecular phylogeny and divergence times of Drosophilia species. Mol. Biol. Evol. 12: 391–404.
SanMiguel P., A. Tikhonov, Y.K Jin, N. Motchoulskaia, D. Zakharov, A. Melake-Berhan, P.S. Springer, K.J. Edwards, M. Lee, Z. Avramova & J.L. Bennetzen, 1996. Nested retrotransposons in the intergenic region of the maize genome. Science 274: 765–768.
Selker, E.U., 1997. Epigenetic phenomena in filamentous fungi: useful paradigm or repeat-induced confusion. Trends Genet. 13: 296–301.
Sheen, F. & R.W. Levis, 1994. Transposition of the LINE-like retrotransposon TART to Drosophila chromosome termini. Proc. Natl. Acad. Sci. USA 91: 12510–12514.
Simonelig, M. & D. Anxolabéhère, 1991. A P element of Scaptomyza pallida is active in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 88: 6102–6106.
Simonelig, M. & D. Anxolabéhère, 1994. P-elements are old components of the Scaptomyza pallida genome. J. Mol. Evol. 38: 232–240.
Smit, A.F.A., 1996. The origin of interspersed repeats in the human genome. Curr. Opin. Genet. Dev. 6: 743–748.
Swofford, D., 1990. PAUP: phylogenic analysis using parsimony Version 4.0. Illinois Natural History Survey, Champaign.
Tabara H., M. Sarkissian, W.G. Kelly, J. Fleenor, A. Grishok, L. Timmons, A. Fire & C.C. Mello, 1999. The rde-1 gene, RNA interference and transposon silencing in C. elegans. Cell 99: 123–132.
Traverse, K.L. & M.L. Pardue, 1988. A spontaneously open ring chromosome of Drosophila melanogaster has acquired He-T DNA at both new telomeres. Proc. Natl. Acad.Sci. USA. 85: 8116–8120.
Wolffe A.P. & M.A. Matzke, 1999. Epigenetics: regulation through repression. Science 286: 481–486.
Witherspoon, D.J., 1999. Selective constraints on P element evolution. Mol. Biol. Evol. 16: 472–478.
Yoder, J., C. Walsh & T. Bestor, 1997. Cytosine methylation and the ecology of intragenomic parasites. Trends Genet. 13: 335–340.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miller, W.J., McDonald, J.F., Nouaud, D. et al. Molecular domestication – more than a sporadic episode in evolution. Genetica 107, 197–207 (1999). https://doi.org/10.1023/A:1004070603792
Issue Date:
DOI: https://doi.org/10.1023/A:1004070603792