Abstract
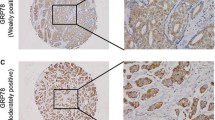
The 78 kDa glucose-regulated stress protein GRP78 is induced by physiological stress conditions such as hypoxia, low pH, and glucose deprivation which often exist in the microenvironments of solid tumors. Activation of this stress pathway occurs in response to several pro-apoptotic stimuli. In vitro studies have demonstrated a correlation between induced expression of GRP78 and resistance to apoptotic death induced by topoisomerase II-directed drugs. We were interested in characterizing this protein in human breast lesions for potential implications in chemotherapeutic intervention. Surgical specimens of human breast lesions and paired normal tissues from the same patients were flash frozen for these studies. Total RNA and/or protein were extracted from these tissues and used in northern and/or western blot analyses, respectively, to quantify the relative expression of GRP78. Northern blot analysis indicated that 0/5 benign breast lesions, 3/5 estrogen receptor positive (ER+) breast tumors, and 6/9 estrogen receptor negative (ER−) breast tumors exhibited overexpression of GRP78 mRNA compared to paired normal tissues, with fold overexpressions ranging from 1.8 to 20. Western blot analyses correlated with these findings since 0/5 benign breast lesions, 4/6 ER+ breast tumors, and 3/3 ER− breast tumors overexpressed GRP78 protein with fold overexpressions ranging from 1.8 to 19. Immunohistochemical analysis of these tissues demonstrated that the expression of GRP78 was heterogeneous among the cells comprising different normal and malignant glands, but confirmed the overexpression of GRP78 in most of the more aggressive ER− tumors. These results suggest that some breast tumors exhibit adverse microenvironment conditions that induce the overexpression of specific stress genes that may play a role in resistance to apoptosis and decreased chemotherapeutic efficacy.
Similar content being viewed by others
References
Feige U, Polla BS: Heat shock proteins: the hsp70 family. Experientia 50: 979–986, 1994
Little E, Ramakrishnan M, Roy B, Gazit G, Lee AS: The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Crit Rev Eukaryot Gene Expr 4: 1–18, 1994
Haas IG: Bip (GRP78), an essential hsp70 resident protein in the endoplasmic reticulum. Experientia 50: 1012–1020, 1994
Munro S, Pelham HRB: An Hsp70-like protein in the ER: identity with the 78 kd glucose regulated protein and immunoglobulin heavy chain binding proteins. Cell 46: 291–300, 1986
Lee AS: Coordinated regulation of a set of genes by glucose and calcium ionophores in mammalian cells. Trends Biochem 12: 20–23, 1987
Dorner AJ, Wasley LC, Kaufman RJ: Protein dissociation from GRP78 and secretion are blocked by depletion of cellular ATP levels. Proc Natl Acad Sci USA 87: 7429–7432, 1990
Rothman JE: Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell 59: 591–601, 1989
Haas IG: BiP - a heat shock protein involved in immunoglobulin heavy chain assembly. Curr Topics Microbiol Immun 167: 71–82, 1991
Gething M-J, Sambrook J: Protein folding in the cell. Nature 355: 33–45, 1992
Kuznetsov G, Chen LB, Nigam SK: Multiple molecular chaperones complex with misfolded large oligomeric glycoproteins in the endoplasmic reticulum. J Biol Chem 272: 3057–3063, 1997
Gething M-J, McCammon K, Sambrook J: Expression of wildtype and mutant forms of influenza hemagglutinin: The role of folding in intracellular transport. Cell 46: 939–950, 1986
Silva AMD, Balch WE, Helenius A: Quality control in the endoplasmic reticulum: folding of vesicular stomatitis virus G protein in cells in vitro. J Cell Biol 111: 857–866, 1990
Vogel JP, Misra LM, Rose MD: Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J Cell Biol 110: 1885–1895, 1990
Nicchitta CV, Blobel G: Luminal proteins of the mammalian endoplasmic reticulum are required to complete protein translocation. Cell 73: 989–998, 1993
Lyman SK, Schekman R: Binding of secretory precursor polypeptides to a translocon subcomplex is regulated by BiP. Cell 88: 85–96, 1997
Knittler MR, Dirks S, Haas IG: Molecular chaperones involved in protein degradation in the endoplasmic reticulum: quantitative interaction of the heat shock cognate protein BiP with partially folded immunoglobulin light chains that are degraded in the endoplasmic reticulum. Proc Natl Acad Sci USA 92: 1764-1768, 1995
Lee AS, Delegeane AM, Baker V, Chow PC: Transcriptional regulation of two genes specifically induced by glucose starvation in a hamster mutant fibroblast cell line. J Biol Chem 258: 597–603, 1983
Lee AS: Mammalian stress response: induction of the glucose regulated protein family. Curr Opin Cell Biol 4: 267–273, 1992
Resendez EJ, Attenello JW, Grafsky A, Chang CS, Lee AS: Calcium ionophore A23187 induces expression of glucoseregulated genes and their heterologous fusion genes. Mol Cell Biol 5: 1212–1219, 1985
Watowich SS, Morimoto RI: Complex regulation of heat shock and glucose-responsive genes in human cells. Mol Cell Biol 8: 393–405, 1988
Dorner AJ, Wasley LC, Raney P, Haugejorden S, Green M, Kaufman RJ: The stress response in Chinese hamster ovary cells. J Biol Chem 265: 22029–22033, 1990
Liu ES, Ou J-h, Lee AS: Brefeldin A as a regulator of grp78 gene expression in mammalian cells. J Biol Chem 267: 7128–7133, 1992
Price BD, Mannheim-Rodman LA, Calderwood SK: Brefeldin A, Thapsigargin, and AlF4-stimulate the accumulation of GRP78 mRNA in a cycloheximide dependent manner, whilst induction by hypoxia is independent of protein synthesis. J Cell Physiol 152: 545–552, 1992
Shen J, Hughes C, Chao C, Cai J, Bartels C, Gessner T, Subjeck J: Coinduction of glucose-regulated proteins and doxorubicin resistance in Chinese hamster cells. Proc Natl Acad Sci USA 84: 3278–3282, 1987
Hughes CS, Shen JW, Subjeck JR: Resistance to etoposide induced by three glucose-regulated stresses in Chinese hamster ovary cells. Cancer Res 49: 4452–4454, 1989
Vichi PJ, Tritton TR: Protection from adriamycin cytotoxicity in L1210 cells by brefeldin A. Cancer Res 53: 5237–5243, 1993
Chatterjee S, Cheng M-F, Berger SJ, Berger NA: Induction of Mr 78, 000 glucose-regulated stress protein in poly(adenosine diphosphate-ribose) polymerase-and nicotinamide adenine dinucleotide-deficient V79 cell lines and its relation to resistance to the topoisomerase II inhibitor etoposide. Cancer Res 54: 4405–4411, 1994
Chatterjee S, Cheng M-F, Berger RB, Berger SJ, Berger NA: Effect of inhibitors of poly(ADP-ribose) polymerase on the induction of GRP78 and subsequent development of resistance to etopiside. Cancer Res 55: 868–873, 1995
Yun J, Tomida A, Nagata K, Tsuruo T: Glucose-regulated stresses confer resistance to VP-16 in human cancer cells through a decreased expression of DNA topoisomerase II. Oncology Res 7: 583–590, 1995
Sacchi CM, Schiaffonati L: The effect of etopiside (VP-16) on mouse L fibroblasts: modulation of stress response, growth and apoptosis genes. Anticancer Res 16: 3659–3664, 1996
Sugawara S, Takeda K, Lee A, Dennert G: Suppression of stress protein GRP78 induction in tumor B/C10ME eliminates resistance to cell mediated cytotoxicity. Cancer Res 53: 6001–6005, 1993
Li L-J, Li X, Ferrario A, Rucker N, Liu ES, Wong S, Gomer CJ, Lee AS: Establishment of a Chinese hamster ovary cell line that expresses GRP78 antisense transcripts and suppresses A23187 induction of both GRP78 and GRP94. J Cell Physiol 153: 575–582, 1992
Koong AC, Chen EY, Lee AS, Brown JM, Giaccia AJ: Increased cytotoxicity of chronic hypoxic cells by molecular inhibition of GRP78 induction. Int J Radiat Oncol Biol Phys 28: 661–666, 1994
Chatterjee S, Hirota H, Belfi CA, Berger SJ, Berger NA: Hypersensitivity to DNA cross-linking agents associated with upregulation of glucose-regulated stress protein GRP78. Cancer Res 57: 5112–5116, 1997
Kennedy KA: Hypoxic cells as specific drug targets for chemotherapy. Anti-Cancer Drug Design 2: 181–194, 1987
Vaupel P, Kallinowski F, Okunieff P: Blood flow, oxygen, and nutrient supply, and metabolic microenvironment of human tumors: A review. Cancer Res 49: 6449–6465, 1989
Pahl HL, Baeuerle PA: The ER-overload response: activation of NF-κB. Trends Biochem Sci 22: 63–67, 1997
Pahl HL, Baeuerle PA: Endoplasmic-reticulum-induced signal transduction and gene expression. Trends Cell Biol 7: 50–55, 1997
Kozutsumi Y, Segal M, Normington K, Gething M-J, Sambrook J: The presence of malfolded proteins in the endo plasmic reticulum signals the induction of glucose-regulated proteins. Nature 332: 462–464, 1988
Patierno SR, Tuscano JM, Kim KS, Landolph JR, Lee AS: Increased expression of the glucose-regulated gene encoding the Mr 78, 000 glucose-regulated protein in chemically and radiation-transformed C3H 10T 1/2 mouse embryo cells. Cancer Res 47: 6220–6224, 1987
Cai JW, Henderson BW, Shen JW, Subjeck JR: Induction of glucose regulated proteins during growth of a murine tumor. J Cell Physiol 154: 229–237, 1993
Jamora C, Dennert G, Lee AS: Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc Natl Acad Sci USA 93: 7690–7694, 1996
Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987
Manning FCR, Xu J, Patierno SR: Transcriptional inhibition by carcinogenic chromate: relationship to DNA damage. Mol Carcinogenesis 6: 156–159, 1992
Lee AS, Delegeane A, Scharff D: Highly conserved glucoseregulated protein in hamster and chicken cells: preliminary characterization of its cDNA clone. Proc Natl Acad Sci USA 78: 4922–4925, 1981
Feinberg AP, Vogelstein B: A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132: 6–13, 1983
Dive C, Hickman JA: Drug target interactions: only the first step in the commitment of a cell to a programmed cell death. Br J Cancer 64: 192–196, 1991
Dive C: Avoidance of apoptosis as a mechanism of drug resistance. J Int Med 242 (Suppl. 740): 139–145, 1997
McCormick TS, McColl KS, Distelhorst CW: Mouse lymphoma cells destined to undergo apoptosis in response to thapsigargin treatment fail to generate a calcium-mediated grp78/grp94 stress response. J Biol Chem 272: 6087–6092, 1997
Kaufmann S: Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemic cells by etoposide, camptothecin, and other cytotoxic anticancer drugs: a cautionary note. Cancer Res 49: 5870–5878, 1989
Liu X, Kim CN, Yang J, Jemmerson R, Wang, X: Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86: 147–157, 1996
Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T: Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280: 1763–1766, 1998
Ciocca DR, Oesterreich S, Chamness GC, McGuire WL, Fuqua S AW: Biological and clinical significance of heat shock protein 27, 000 (Hsp27): a Review. J Natl Cancer Inst 85: 1558–1570, 1993
Edwards DP, Adams DJ, McGuire WL: Estradiol stimulates synthesis of a major intracellular protein in a human breast cancer cell line (MCF-7). Breast Cancer Res Treat 1: 209–223, 1981
Ramachandran C, Catelli MG, Schneider W, Shyamala G: Estrogenic regulation of uterine 90-kilodalton heat shock protein. Endocrinology 123: 956–961, 1988
Storm FK, Gilchrist KW, Warner TF, Mahvi DM: Distribution of Hsp-27 and HER-2/neu in in situ and invasive ductal breast carcinomas. Ann Surg Oncol 2: 43–48, 1995
Storm FK, Mahvi DM, Gilchrist KW: Lack of association between tumor necrosis and Hsp-27 expression in primary breast cancer. J Surg Oncol 61: 14–16, 1996
Fuqua SAW, Oesterreich S, Hilsenbeck SG, Von Hoff DD, Eckardt J, Osborne, CK: Heat shock proteins and drug resistance. Breast Cancer Res Treat, 32: 67-71, 1994
Oesterreich S, Weng C-N, Qiu M, Hilsenbeck SG, Osborne CK, Fuqua SAW: The small heat shock protein Hsp27 is correlated with growth and drug resistance in human breast cancer cell lines. Cancer Res 53: 4443-4448, 1993
Ciocca DR, Fuqua SAW, Lock-Lim S, Toft DO, Welch, WJ, McGuire WL: Response of human breast cancer cells to heat shock and chemotherapeutic drugs. Cancer Res 52: 3648-3654, 1992
Ciocca DR, Green S, Elledge RM, Clark GM, Pugh R, Ravdin P, Lew D, Martino S, Osborne CK: Heat shock proteins Hsp27 and Hsp70: Lack of correlation with response to tamoxifen and clinical course of disease in estrogen receptor-positive metastatic breast cancer (A Southwest Oncology Group Study). Clin Cancer Res 5: 1263-1266, 1998
Lin ZP, Boller YC, Amer SM, Russell RL, Pacelli KA, Patierno SR, Kennedy KA: Prevention of brefeldin A-induced resistance to teniposide by the proteasome inhibitor MG-132: involvement of NF-κB activation in drug resistance. Cancer Res 58: 3059–3065, 1998
Lin ZP, Patierno SR, Kennedy KA: Activation of NF-κB is involved in hypoxia-induced drug resistance to topoisomerase II-directed agents. (submitted), 1999
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fernandez, P.M., Tabbara, S.O., Jacobs, L.K. et al. Overexpression of the glucose-regulated stress gene GRP78 in malignant but not benign human breast lesions. Breast Cancer Res Treat 59, 15–26 (2000). https://doi.org/10.1023/A:1006332011207
Issue Date:
DOI: https://doi.org/10.1023/A:1006332011207