Abstract
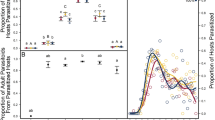
With reciprocal rearing experiments, we tested the hypothesis that adaptive differences in host-use traits among soapberry bug populations have a genetic basis. These experiments were conducted with two host races from Florida, an ‘ancestral-type’ one on a native host plant species and a ‘derived’ one on a recently introduced plant species (colonized mainly post-1950), on whose seed crops this insect depends for growth and reproduction. Compared to the native host species, the introduced host produces larger seed crops over a much briefer annual period. Its seeds are also significantly higher in lipids and lower in nitrogen. The bug populations exhibit greater juvenile survivorship on their ‘home’ hosts; that is, the derived population survives better on seeds of the introduced host than does its ancestral-type counterpart, and vice versa. Regardless of the rearing host, populations from the introduced host lay much smaller eggs, and fecundity measures show a more complex pattern than does survivorship: the ancestral-type population produces eggs at the same rate on each host, while the derived population is less fecund on the native host and exhibits enhanced fecundity on the introduced host. These results indicate that the population differences are evolved rather than host-induced. They appear to be adaptive responses to host differences in the spatial and temporal distribution of seed availability and nutritional quality, and show that increased performance on the alien host has evolved with surprising speed and magnitude, with concomitant reductions in performance on the original host.
Similar content being viewed by others
References
Aldrich, J.R., Carroll, S.P., Lusby, W.R., Thompson, M.J., Kochansky, J.P. and Waters, R.M. (1990) Sap-indaceae, cyanolipids, and bugs. J. Chem. Ecol. 16, 199-210.
Ananthakrishnan, T.N., Raman, K. and Sanjayan, K.P. (1982) Comparative growth rate, fecundity and behavioral diversity of the dusky cotton bug, Oxycarenus hyalinipennis Costa (Hemiptera: Lygaeidae) on certain malvaceous plants. Proc. Indian Natl. Acad. Sci. 48B, 577-584.
Bishop, J.A., Cook, L.M. and Muggleton, J. (1977) The response of two species of moths to industrialization in northwest England. Phil. Trans. Roy. Soc. Lond. B 281, 517-540.
Bush, G.L. (1969) Sympatric host race formation and speciation in frugivorous flies of the genus Rhagoletis (Diptera: Tephritidae). Evolution 23, 237-251.
Carroll, S.P. (1988) Contrasts in reproductive ecology between temperate and tropical populations of Jadera haematoloma (Rhopalidae), a mate-guarding hemipteran. Ann. Entomol. Soc. Am. 81, 54-63.
Caroll, S.P. (1991) The adaptive significance of mate guarding in the soapberry bug Jadera haemataloma (Hemiptera: Rhopalidae). J. Insect Behav. 4, 509-530.
Carroll, S.P. and Boyd, C. (1992) Host race radiation in the soapberry bug: Natural history, with the history. Evolution 46, 1052–1069.
Carroll, S.P. and Loye, J.E. (1987) Specialization of Jadera species (Hemiptera: Rhopalidae) on seeds of the Sapindaceae, and coevolution of defense and attack. Ann. Entomol. Soc. Am. 80, 373-378.
Carroll, S.P., Dingle, H. and Klassen, S.P. (1997) Genetic differentiation of fitness-associated traits among rapidly evolving populations of the soapberry bug. Evolution 51, 1182-1188.
Derr, J.A., Alden, B. and Dingle, H. (1981) Insect life histories in relation to migration, body size, and host plant array: A comparative study of Dysdercus. J. Anim. Ecol. 50, 181-193
Diehl, S.R. and Bush, G.L. (1989) The role of habitat preference in adaptation and speciation. In Speciation and its Consequences (D. Otte and J. Endler, eds), pp. 345-365. Sinauer Associates, Sunderland, MA.
Dingle, H. and Winchell, R. (1997) Juvenile-hormone as a mediator of plasticity in insect life histories. Arch. Insect Biochem. Physiol. 35, 359-373.
Gibbs, H. and Grant, P.R. (1987) Oscillating selection on Darwin's finches. Nature 327, 511-513.
Grant, P.R. and Grant, B.R. (1995) Predicting microevolutionary responses to directional selection on heritable variation. Evolution 49, 241-251.
McNeilly, T. (1967) Evolution in closely adjacent plant populations. III. Agrostis tenuis on a small copper mine. Heredity 23, 99-108.
Pashley, D.P. (1986) Host-associated genetic differentiation in fall armyworm (Lepidoptera: Noctuidae): A sibling species complex? Ann. Entomol. Soc. Am. 79, 898-904.
Pashley, D.P. (1988) Quantitative genetics, development, and physiological adaptation in host strains of the fall armyworm. Evolution 42, 93-102.
Pegueroles, G., Papceit, M., Quintana, A., Guilleén, A., Prevosti, A. and Serra, L. (1995) An experimental study of evolution in progress: Clines for quantitative traits in colonizing and Palearctic populations of Drosophila. Evol. Ecol. 9, 463-465.
Phillips, P.A. and Barnes, M.M. (1975) Host race formation among sympatric apple, walnut, and plum populations of the codling moth, Laspeyresia pomonella. Ann. Entomol. Soc. Am. 68, 1053-1060.
Prokopy, R.J., Diehl, S.R. and Cooley, S.S. (1988) Behavioral evidence for host races in Rhagoletis pomonella flies. Oecologia 76, 138-147.
Rausher, M.D. (1982) Population di.erentiation in Euphydryas editha butterflies: Larval adaptation to different hosts. Evolution 36, 581-590.
Siegler, D.S. and Kawahara, W. (1976) New reports of cyanolipids from sapindaceous plants. Biochem. Syst. Ecol. 4, 263-265.
Singer, M.C., Ng, D. and Thomas, C.D. (1988) Heritability of oviposition preference and its relationship to o.spring performance within a single insect population. Evolution 42, 977-985.
Singer, M.C., Thomas, C.D. and Parmesan, C. (1993) Rapid human-induced evolution of insect-host associations. Nature 366, 681-683.
Smith, D.C. (1988) Heritable divergence of Rhagoletis pomonella host races by seasonal asynchrony. Nature 336, 66-67.
Southwood, T.R.E. (1988) Tactics, strategies and templates. Oikos 52, 3-18.
Tabashnik, B.E. (1983) Host range evolution: The shift from native legume hosts to alfalfa by the butterfly, Colias eriphyle. Evolution 37, 150-162.
Via, S. (1984) The quantitative genetics of polyphagy in an insect herbivore. I. Genotype-environment interaction in larval performance on different host plants. Evolution 38, 881-895.
Via, S. (1986) Quantitative genetic analysis of feeding and oviposition behavior in the polyphagous leafminer Liriomyza sativa. In Evolutionary Genetics of Invertebrate Behavior (M.D. Huettel, ed.), pp. 185-196. Plenum Press, New York.
Via, S. (1991a) Specialized host plant performance of pea aphid clones is not altered by experience. Ecology 72, 1420-1427.
Via, S. (1991b) The genetic structure of host plant adaptation in a spatial patchwork-demographic variability among reciprocally transplanted pea aphid clones. Evolution 45, 827-852. 968 Carroll et al.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Carroll, S.P., Klassen, S.P. & Dingle, H. Rapidly evolving adaptations to host ecology and nutrition in the soapberry bug. Evolutionary Ecology 12, 955–968 (1998). https://doi.org/10.1023/A:1006568206413
Issue Date:
DOI: https://doi.org/10.1023/A:1006568206413