Abstract
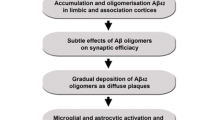
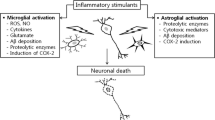
Alzheimer's disease (AD) is a progressive, neurodestructive process of the human neocortex, characterized by the deterioration of memory and higher cognitive function. A progressive and irreversible brain disorder, AD is characterized by three major pathogenic episodes involving (a) an aberrant processing and deposition of β-amyloid precursor protein (βAPP) to form neurotoxic beta-amyloid (βA) peptides and an aggregated insoluble polymer of βA that forms the senile plaque, (b) the establishment of intraneuronal neuritic tau pathology yielding widespread deposits of agyrophilic neurofibrillary tangles (NFT) and (c) the initiation and proliferation of a brain-specific inflammatory response. These three seemingly disperse attributes of AD etiopathogenesis are linked by the fact that proinflammatory microglia, reactive astrocytes and their associated cytokines and chemokines are associated with the biology of the microtubule associated protein tau, βA speciation and aggregation. Missense mutations in the presenilin genes PS1 and PS2, implicated in early onset familial AD, cause abnormal βAPP processing with resultant overproduction of βA42 and related neurotoxic peptides. Specific βA fragments such as βA42 can further potentiate proinflammatory mechanisms. Expression of the inducible oxidoreductase cyclooxygenase-2 and cytosolic phospholipase A2 (cPLA2) are strongly activated during cerebral ischemia and trauma, epilepsy and AD, indicating the induction of proinflammatory gene pathways as a response to brain injury. Neurotoxic metals such as aluminum and zinc, both implicated in AD etiopathogenesis, and arachidonic acid, a major metabolite of brain cPLA2 activity, each polymerize hyperphosphorylated tau to form NFT-like bundles. Further, epidemiological and longitudinal studies have identified a reduced risk for AD in patients (<70 yrs) previously treated with non-steroidal anti-inflammatory drugs for non-CNS afflictions that include arthritis. This review will focus on the interrelationships between the mechanisms of PS1, PS2 and βAPP gene expression, tau and βA deposition and the induction, regulation and proliferation in AD of the neuroinflammatory response. Novel therapeutic interventions in AD are discussed.
Similar content being viewed by others
REFERENCES
Braak, H. and Braak, E. 1997. Staging of Alzheimer-related cortical destruction. Int. Psychogeriatr. 257:61.
Selkoe, D. J. 1994. Normal and abnormal biology of the beta-amyloid precursor protein. Ann. Rev. Neurosci. 17:489-517.
Ray, W. J., Ashall, F., and Goate, A. M. 1998. Molecular pathogenesis of sporadic and familial forms of Alzheimer's disease. Molec. Med. Today 4:151-157.
St. George-Hyslop, P. H. 2000. Molecular genetics of Alzheimer's disease. Biol. Psychiatry 47:183-199.
McGeer, P. L. and McGeer, E G. 1995. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res. Rev. 21:195-218.
McGeer, P. L., Schulzer, M., and McGeer, E. G. 1996. Arthritis and antiinflammatory agents as possible protective factors for Alzheimer's disease; a review of 17 epidemiologic studies. Neurology 47:425-432.
Emmerling, M. R., Moore, C. J., Doyle, P. D., Carroll, R. T., and Davis, R. E. 1993. Phospholipase A2 activation influences the processing and secretion of the amyloid precursor protein. Biochem. Biophys. Res. Commun. 197:292-297.
Stephenson, D., Rash, K., Smalstig, B., Roberts, E., Johnstone, E., Sharp, J., Panetta, J., Little, S., Kramer, R., and Clemens, J. 1999. Cytosolic phospholipase A2 is induced in reactive glia following different forms of neurodegeneration. Glia 27:110-128.
Halliday, G., Robinson, S. R., Shepherd, C., and Kril, J. 2000. Alzheimer's disease and inflammation: a review of cellular and therapeutic mechanisms. Clin. Exp. Pharmacol. Physiol. 27:1-8.
Lukiw, W. J. and Bazan, N. G. 1997. Cyclooxygenase-2 RNA message abundance, stability, and hypervariability in sporadic Alzheimer neocortex. PMID: 9452008; UI: 98112387. J. Neurosci. Res. 50:937-945.
Lukiw, W. J. and Bazan, N. G. 1998. Strong nuclear factor-kappaB-DNA binding parallels cyclooxygenase-2 gene transcription in aging and in sporadic Alzheimer's disease superior temporal lobe neocortex. J. Neurosci. Res. 53:583-592.
Pasinetti, G. M. and Aisen, P. S. 1998. Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer's disease brain. Neuroscience 87:319-324.
Bazan, N. G. 1998. The neuromessenger platelet-activating factor in plasticity and neurodegeneration. Prog. Brain Res. 118:281-291.
Rockenstein, E. M., McConlogue, L., Tan, H., et al. 1995. Levels and alternative splicing of amyloid beta protein precurser (APP) transcripts in brains of APP transgenic mice and humans with Alzheimer's disease. JBC 270:28257-28267.
Mattson, M. P. 1997. Cellular actions of beta-amyloid precurser protein and its soluble and fibrillogenic derivatives. Physiol. Rev. 77:1081-1132.
Alzheimer, A., Stelzmann, R. A., Schnitzlein, H. N., et al. 1995. An English translation of Alzheimer's 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde” Clin. Anat. 8:429-431.
Hla, T. and Neilson, K. 1992. Human cyclooxygenase-2 cDNA. Proc. Natl. Acad. Sci. 89:7384-7388.
Kosaka, T., Miyata, A., Ihara, H., et al. 1994. Characterization of the human gene (PTGS2) encoding prostaglandinendoperoxide 2 synthase. Eur. J. Biochem. 221:889-897.
Lukiw, W. J., Pelaez, R. P., Martinez J., and Bazan, N. G. 1998. Budesonide epimer R or dexamethasone selectively inhibit platelet-activating factor-induced or interleukin 1betainduced DNA binding activity of cis-acting transcription factors and cyclooxygenase-2 gene expression in human epidermal keratinocytes. Proc. Natl. Acad. Sci. USA 95:3914-3919.
Nogawa, S., Zhang, F., Ross, M. E., et al. 1997. COX-2 gene expression in neurons contributes to ischemic brain damage. J. Neurosci. 17:2746-2755.
Ristmaki, A., Narko, K., and Hla, T. 1996. Down-regulation of cytokine-induced cyclooxygenase-2 transcript isoforms by dexamethasone: evidence for post-transcriptional regulation. Biochem. J. 318:325-331.
Bazan, N. G., Fletcher, B. S., Herschman, H. R., et al. 1994. Platelet-activating factor and retinoic acid synergistically activate the inducible prostaglandin synthase gene. Proc. Natl. Acad. Sci. USA 91:5252-5256.
Marcheselli, V. L. and Bazan, N. G. 1996. Sustained induction of prostaglandin endoperoxide synthase-2 by seizures in hippocampus. Inhibition by a platelet-activating factor antagonist. J. Biol. Chem. 271:24794-24799.
Dickson, D. W., Lee, S. C., Mattiace, L. A., et al. 1993. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer's disease. Glia 7:75-83.
Lee, S. C., Dickson, D. W., and Brosnan, C. F. 1995. Interleukin-1, nitric oxide and reactive astrocytes. Brain Behav. Immun. 9:345-354.
Sen, R. and Baltimore, D. 1986. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 46:705-716.
Baeuerle, P. A. and Henkel, T. 1994. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 12:141-179.
Kaltschmidt, C., Kaltschmidt, B., and Neumann, H. 1994. Constitutive NF-kappa B activity in neurons. Mol. Cell. Biol. 14:3981-3992.
Kaltschmidt, B., Uherek, M., Volk, B., et al. 1997. Transcription factor NF-kappaB is activated in primary neurons by amyloid beta peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc. Natl. Acad. Sci. USA 94:2642-2647.
Stylianou, E. and Saklatvala, J. 1998. Interleukin-1. Int. J. Biochem. Cell Biol. 30:1075-1079.
Kaltschmidt, C., Kaltschmidt, B., and Baeuerle, P. A. 1993. Brain synapses contain inducible forms of the transcription factor NF-kappa B. Mech. Dev. 43:135-147.
Suzuki, T., Mitake, S., Okumura-Noji, K., et al. 1997. Presence of NF-kappaB-like and IkappaB-like immunoreactivities in postsynaptic densities. Neuroreport 8:2931-2935.
Stylianou, E., O'Neill, L. A., Rawlinson, L., et al., 1992. Interleukin 1 induces NF-kappa B through its type I but not its type II receptor in lymphocytes. J. Biol. Chem. 267:15836-15841.
Traenckner, E. B., Pahl, H. L., Henkel, T., et al. 1995. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 14:2876-2883.
Fujita, T., Nolan, G. P., Ghosh, S., et al. 1992. Independent modes of transcriptional activation by the p50 and p65 subunits of NF-kappa B. Genes Dev. 6:775-787.
Verma, I. M., Stevenson, J. K., Schwarz, E. M., et al. 1995. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 15:2723-2735.
Boissiere, F., Hunot, S., Faucheux, B., et al. 1997. Nuclear translocation of NF-kappaB in cholinergic neurons of patients with Alzheimer's disease. Neuroreport 8:2849-2852.
Baeuerle, P. A. and Baltimore, D. 1996. NF-κB: Ten years after. Cell 87:13-20.
Grilli, M., Goffi, F., Memo, M., et al. 1996. Interleukin-1beta and glutamate activate the NK-kappaB/Rel binding site from the regulatory region of the amyloid precursor protein gene in primary neuronal cultures. J. Biol. Chem. 271:15002-15007.
Newton, R., Kuitert, L. M., Bergmann, M., et al. 1997. Evidence for involvement of NF-kappaB in the transcriptional control of COX-2 gene expression by IL-1beta. Biochem. Biophys. Res. Commun. 237:28-32.
Newton, R., Kuitert, L. M., Slater, D. M., et al. 1997. Cytokine induction of cytosolic phospholipase A2 and cyclooxygenase-2 mRNA is suppressed by glucocorticoids in human epithelial cells. Life Sci. 60:67-78.
Newton, R., Stevens, D. A., Hart, L. A., et al. 1997. Superinduction of COX-2 mRNA by cycloheximide and interleukin-1 beta involves increased transcription and correlates with increased NF-kappaB and JNK a c t i v a t i o n. FEBS Lett. 418:135-138.
Lukiw, W. J., LeBlanc, H. J., Carver, L. A., McLachlan, D. R., and Bazan, N. G. 1998. Run-on gene transcription in human neocortical nuclei. Inhibition by nanomolar aluminum and implications for neurodegenerative disease. J. Mol. Neurosci. 11:67-78.
Grilli, M., Pizzi, M., Memo, M., et al. 1996. Neuroprotection by aspirin and sodium salicylate through blockade of NF-kappa B activation. Science 274:1383-1385.
Guerrini, L., Molteni, A., Wirth, T., et al. 1997. Glutamatedependent activation of NF-kappaB during mouse cerebellum development. J. Neurosci. 17:6057-6063.
Brostjan, C., Anrather, J., Csizmadia, V., et al. 1996. Glucocorticoid-mediated repression of NFkappaB activity in endothelial cells does not involve induction of IkappaBalpha synthesis. J. Biol. Chem. 271:19612-19616.
Lukiw, W. J., Martinez, J., Pelaez, R. P., and Bazan, N. G. 1999. The interleukin-1 type 2 receptor gene displays immediate early gene responsiveness in glucocorticoid-stimulated human epidermal keratinocytes. J. Biol. Chem. 274:8630-8638.
Behl, C., Davis, J. B., Lesley, R., et al. 1994. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell 77:817-827.
Hensley, K., Butterfield, D. A., Hall, N., et al. 1996. Reactive oxygen species as causal agents in the neurotoxicity of the Alzheimer's disease-associated amyloid beta peptide. Ann NY Acad. Sci. 786:120-134.
O'Neill, L. A. and Kaltschmidt, C. 1997. NF-kappaB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 20:252-258.
Rong, Y. and Baudry, M. 1996. Seizure activity results in a rapid induction of nuclear factor-kappa B in adult but not juvenile rat limbic structures. J. Neurochem. 67:662-668.
Salminen, A., Liu, P. K., and Hsu, C. Y. 1995. Alteration of transcription factor binding activities in the ischemic rat brain. Biochem. Biophys. Res. Commun. 212:939-944.
Colangelo, V., Lukiw, W. J., Gordon, W. C., et al. 1999. COX-2 as a mediator of cerebral ischemic tolerance. ASN Meeting abstract 95-S30B.
Lukiw, W. J. and Bazan, N. G. 2000. Presenilin-1 (PS1) and cyclooxygenase-2 (COX-2) genes exhibit coordinated transcription in experimental epileptogenesis. American Epilepsy Society Abstract. K.05.
Terai, K., Matsuo, A., McGeer, E. G., et al. 1996. Enhancement of immunoreactivity for NF-kappaB in human cerebral infarctions. Brain Res. 739:343-349.
Kaltschmidt, B., Baeuerle, P. A., and Kaltschmidt, C. 1993. Potential involvement of the transcription factor NF-kappa B in neurological disorders. Mol. Aspects Med. 14:171-190.
Hunot, S., Brugg, B., Ricard, D., et al. 1997. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease. Proc. Natl. Acad. Sci. USA 94:7531-7536.
Kitamura, Y., Shimohama, S., Ota, T, et al. 1997. Alteration of transcription factors NF-kappaB and STAT1 in Alzheimer's disease brains. Neurosci. Lett. 237:17-20.
Liu, M., Kumar, K. U., Pater, M. M., et al. 1997. Dual NFl-requiring effect of human neurotropic JC virus composite pentanucleotide repeat elements on early and late viral gene expression. Virology 227:7-12.
Perkins, D. J. and Kniss, D. A. 1997. Tumor necrosis factoralpha promotes sustained cyclooxygenase-2 expression: attenuation by dexamethasone and NSAIDS. Prostaglandins 54:727-743.
Neve, R. L., Rogers, J., and Higgins, G. A. 1990. The Alzheimer amyloid precursor-related transcript lacking the beta/A4 sequence is specifically increased in Alzheimer's disease brain. Neuron 5:329-338.
Lukiw, W. J., Rogaev, E. I., Wong, L., et al. 1994. Protein-DNA interactions in the promoter region of the amyloid precursor protein (APP) gene in human neocortex. Brain Res. Mol. Brain Res. 22:121-131.
Li, J., Xu, M., Zhou, H., et al. 1997. Alzheimer presenilins in the nuclear membrane, interphase kinetochores, and centrosomes suggest a role in chromosome segregation. Cell 90:917-927.
Shirotani, K., Takahashi, K., Ozawa, K., et al. 1997. Determination of a cleavage site of presenilin 2 protein in stably transfected SH-SY5Y human neuroblastoma cell lines. Biochem. Biophys. Res. Commun. 240:728-731.
Chui, D. H., Shirotani, K., Tanahashi, H., et al. 1998. Both Nterminal and c-terminal fragments of presenilin 1 colocalize with neurofibrillary tangles in neurons and dystrophic neurites of senile plaques in Alzheimer's disease. J. Neurosci. Res. 53:99-106.
Spencer, A. G., Woods, J. W., Arakawa, T., et al. 1998. Subcellular localization of a prostaglandin endoperoxide H synthases-1 and-2 by immunoelectron microscopy. J. Biol. Chem. 273:9886-9893.
Rogaev, E. I., Bazan, N. G., and Lukiw, W. J. 1998. Common regulatory elements in the promoters of genes linked to familial or sporadic Alzheimer's disease (AD). SFN 28th Annual Meeting; Los Angeles, CA.
Lukiw, W. J., Rogaev, E. I., and Bazan, N. G. 2000. Analysis of transcriptional regulatory mechanisms in nine genes associated with Alzheimer's disease (AD). J. Neurosci. Res. (Submitted).
Weggen, S., Diehlmann, A., Buslei, R., et al. 1998. Prominent expression of presenilin-1 in senile plaques and reactive astrocytes in Alzheimer's disease brain. Neuroreport 9:3279-3283.
Tanimukai, H., Imaizumi, K., Kudo, T., et al. 1998. Alzheimerassociated presenilin-1 gene is induced in gerbil hippocampus after transient ischemia. Mol. Brain Res. 54:212-218.
Holcomb, L., Gordon, M. N., McGowan, E., et al. 1998. Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat. Med. 4:97-100.
Goldgaber, D., Harris, H. W., Hla, T., et al. 1989. Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc. Natl. Acad. Sci. USA 86:7606-7610.
Tong, W., Shah, D., Xu, J., Diehl, J. A., Hans, A., Hannink, M., and Sun, G. Y. 1999. Involvement of lipid mediators on cytokine signaling and induction of secretory phospholipase A2 in Immortalized astrocytes (DITNC). J. Mol. Neurosci. 12:89-99.
Shimizu, T. and Wolfe, L. S. 1990. Arachidonic acid cascade and signal transduction. J. Neurochem. 55:1-15.
Murakami, M., Nakatani, Y., Atsumi, G. Inoue, K., and Kudo, I. 1997. Regulatory functions of phospholipase A2. Crit. Rev. Immunol. 17:225-228.
Gijon, M. A. and Leslie, C. C. 1999. Regulation of arachidonic acid release and cytosolic phospholipase A2 activation. J. Leukoc. Biol. 65:330-336.
Hirabayashi, T., Kume, K., Hirose, K., Yokomizo, T., Iino, M., Itoh, H., and Shimizu, T. 1999. Critical duration of intracellular Ca2+response required for continuous translocation and activation of cytosolic phospholipase A2. J. Biol. Chem. 274:5163-5169.
Suzuki, N., Ishizaki, J., Yokota, Y., Higashino, K., Ono, T., Ikeda, M., Fujii, N., Kawamoto, K., and Hanasaki, K. 2000. Structures, enzymatic properties, and expression of novel human and mouse secretory phospholipase A(2)s. J. Biol. Chem. 275:5785-5793.
Peplow, P. V. 1999. Regulation of platelet-activating factor (PAF) activity in human diseases by phospholipase A2 inhibitors, PAF acetylhydrolases, PAF receptor antagonists and free radical scavengers. Prostaglandins Leukot. Essent. Fatty Acids 61:65-82.
Snowdon, D. A., Greiner, L. H., Mortimer, J. A., et al. 1997. Brain infarction and the clinical expression of Alzheimer disease. JAMA 277:813-817.
Mirra, S. S. and Gearing, M. 1997. Brain infarction and the clinical expression of Alzheimer's disease. JAMA 278:113.
Heyman, A., Fillenbaum, G. G., Welsh-Bohmer, K. A., et al. 1998. Cerebral infarcts in patients with autopsy-proven Alzheimer's disease: CERAD, part XVIII. Consortium to Establish a Registry for Alzheimer's Disease. Neurology 51:159-162.
Colangelo, V., Lukiw, W. J., Gordon, W. C., et al. 1999. COX-2 as a mediator of cerebral ischemic tolerance. ASN Meeting abstract 95-S30B.
Bing, G., Wilson, B., Hudson, P., Jin, L., Feng, Z., Zhang, W., Bing, R., and Hong, J. S. 1997. A single dose of kainic acid elevates the levels of enkephalins and activator protein-1 transcription factors in the hippocampus for up to 1 year. Proc. Natl. Acad. Sci. USA 94:9422-9427.
Lukiw, W. J., Tu, B., Marcheselli, V. L., and Bazan, N. G. 1999. Delayed hippocampal activation of the transcription factor NF-κB in experimental epileptogenesis. AES 53rd Annual Meeting; Orlando.
Frederikse, P. H. and Zigler, J. S. Jr. 1998. Presenilin expression in the ocular lens. Current Eye Research 17:947-952.
Benzing, W. C., Wujek, J. R., Ward, E. K., Shaffer, D., Ashe, K. H., Younkin, S. G., and Brunden, K. R. 1999. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol. Aging 20:581-589.
Mantyh, P. W., Ghilardi, J. R., Rogers, S., DeMaster, E., Allen, C. J., Stimson, E. R., and Maggio, J. E. 1993. Aluminum, iron, and zinc ions promote aggregation of physiological concentrations of beta-amyloid peptide. J. Neurochem. 61:1171-1174.
Lukiw, W. J. 1997. Aluminum in Alzheimer's disease. Pages 113-126 in Yasui, M., Strong, M., Ota, K., and Verity, M. A. (eds.), Mineral and Metal Neurotoxicology, CRC Press, Boca Raton Florida.
Campbell, A., Kumar, A., La Rosa, F. G., Prasad, K. N., and Bondy, S. C. 2000. Aluminum increases levels of beta-amyloid and ubiquitin in neuroblastoma but not in glioma cells. Proc. Soc. Exp. Biol. Med. 223:397-402.
Stephenson, D. T., Lemere, C. A., Selkoe, D. J., and Clemens, J. A. 1996. Cytosolic phospholipase A2 (cPLA2) immunoreactivity is elevated in Alzheimer's disease brain. Neurobiol. Dis. 3:51-63.
King, M. E., Ahuja, V., Binder, L. I., and Kuret, J. 1999. Ligand-dependent tau filament formation: implications for Alzheimer's disease progression. Biochemistry 38:14851-14859.
Yates, S. L., Burgess, L. H., Kocsis-Angle, J., Antal, J. M., Dority, M. D., Embury, P. B., Piotrkowski, A. M., and Brunden, K. R. 2000. Amyloid beta and amylin fibrils induce increases in proinflammatory cytokine and chemokine production by THP-1 cells and murine microglia. J. Neurochem. 74:1017-1025.
Abdel-Ghany, M., el-Sebae, A. K., and Shalloway, D. 1993. Aluminum-induced nonenzymatic phospho-incorporation into human tau and other proteins. J. Biol. Chem. 268:11976-11981.
Bush, A. I., Pettingell, W. H. Jr., Paradis, M. D., and Tanzi, R. E. 1994. Modulation of A beta adhesiveness and secretase site cleavage by zinc. J. Biol. Chem. 269:12152-12158.
Moir, R. D., Atwood, C. S., Huang, X., Tanzi, R. E., and Bush, A. I. 1999. Mounting evidence for the involvement of zinc and copper in Alzheimer's disease. Eur. J. Clin. Invest. 29:569-570.
Exley, C. 1999. A molecular mechanism of aluminium-induced Alzheimer's disease? J. Inorg. Biochem. 76:133-140.
Christen, Y. 2000. Oxidative stress and Alzheimer disease. Am. J. Clin. Nutr. 71:621S-629S.
Cuajungco, M. P. and Lees, G. J. 1997. Zinc metabolism in the brain: relevance to human neurodegenerative disorders. Neurobiol. Dis. 4:137-169.
Savory, J., Huang, Y., Wills, M. R., and Herman, M. M. 1998. Reversal by desferrioxamine of tau protein aggregates following two days of treatment in aluminuminduced neurofibrillary degeneration in rabbit: implications for clinical trials in Alzheimer's disease. Neurotoxicology 19:209-214.
Suh, S. W., Jensen, K. B., Jensen, M. S., Silva, D. S., Kesslak, P. J., Danscher, G., and Frederickson, C. J. 2000. Histochemically reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer's diseased brains. Brain Res. 852:274-278.
Walker, P. R., LeBlanc, J., and Sikorska, M. 1989. Effects of aluminum and other cations on the structure of brain and liver chromatin. Biochemistry 28:3911-3915.
Klegeris, A. and McGeer, P. L. 2000. Interaction of various intracellular signaling mechanisms involved in mononuclear phagocyte toxicity toward neuronal cells. J. Leukoc. Biol. 67:127-133.
Ceballos-Picot, I., Merad-Boudia, M., Nicole, A., Thevenin, M., Hellier, G., Legrain, S., and Berr, C. 1996. Peripheral antioxidant enzyme activities and selenium in elderly subjects and in dementia of Alzheimer's type-place of the extracellular glutathione peroxidase. Free Radic. Biol. Med. 20:579-587.
Barinaga, M. 1999. An immunization against Alzheimer's? Science 285:175-177.
Kramer, R. M., Stephenson, D. T., Roberts, E. F., and Clemens, J. A. 1996. Cytosolic phospholipase A2 (cPLA2) and lipid mediator release in the brain. J. Lipid Mediat. Cell Signal 14:3-7.
Lukiw, W. J., Rodriguez de Turco, E. B., and Bazan, N. G. 2000. Brain phospholipase A2 (PLA2) in cerebral injury, neural degeneration and Alzheimer's disease. Biochemica et Biophysica Acta (Submitted).
Combs, C. K., Johnson, D. E., Karlo, J. C., Cannady, S. B., and Landreth, G. E. 2000. Inflammatory mechanisms in Alzheimer's disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPAR gamma agonists. J. Neurosci. 20:558-567.
Sutton, E. T., Thomas, T., Bryant, M. W., Landon, C. S., Newton, C. A., and Rhodin, J. A. 1999. Amyloid-beta peptide induced inflammatory reaction is mediated by the cytokines tumor necrosis factor and interleukin-1. J. Submicrosc. Cytol. Pathol. 31:313-323.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lukiw, W.J., Bazan, N.G. Neuroinflammatory Signaling Upregulation in Alzheimer's Disease. Neurochem Res 25, 1173–1184 (2000). https://doi.org/10.1023/A:1007627725251
Issue Date:
DOI: https://doi.org/10.1023/A:1007627725251
- Alzheimer's disease
- AP1
- beta-amyloid precursor protein (βAPP)
- beta-amyloid (βA) peptide
- ethylene diamine tetra acetic acid (EDTA)
- human hippocampal CA1
- inflammatory response
- microtubule associated protein (MAP)
- nuclear factor for kappa B (NF-κB)
- neurofibrillary tangle (NFT)
- platelet-activating factor (PAF)
- presenilin-1 (PS1)
- synaptic signaling
- tau protein