Abstract
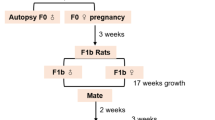
Spirulina maxima, provided by Sosa Texcoco Company (México City), was administered to mice of both sexes in a fertility study, at concentrations of 0, 10, 20 and 30% incorporated into the diet. Males were fed for nine weeks while females, for two weeks, and feeding continued during the mating period and gestation. On the other hand, in a peri- and postnatal study, the alga was given only to females at the same concentrations from day 15 of gestation until day 21 post-partum. Treatments were not associated with any adverse effect on reproductive performance, pregnancy rate, number of corpora lutea, resorptions or number of live or dead fetuses. There was no increase in the number of abnormal pups at caesarean section. Length of gestation, parturition status, and litter values were unaffected by treatment. However, there was a statistically significant reduction in bodyweight and survival rate on postnatal days 0–4 at the high dose group in the peri- and postnatal study. The reproductive performance of F1 generation was normal in all groups. We conclude that S. maxima is not toxic to reproduction.
Similar content being viewed by others
References
Becker EW (1980) Comparative toxicological studies with algae in India, Thailand and Peru. In Shelef G, Soeder CJ (eds), Algae Biomass. Production and Use. Elsevier/North-Holland, Amsterdam, 767–786; 565–574.
Becker EW (1995) Microalgae. Biotechnology and Microbiology. Cambridge University Press, Cambridge, 293 pp.
Belay A, Toshimitsu K, Yoshimichi O (1997) Spirulina (Arthospira): potential application as an animal feed supplement. J. appl. Phycol. 18: 303–311.
Belay A, Yoshimichi O, Kayuzuki M, Shimamatsu H (1993) Current knowledge on potential health benefits of Spirulina. J. appl. Phycol. 5: 235–241.
Chamorro G, Herrera G, Salazar M, Salazar S, Ulloa V (1988) Subchronic toxicity study in rats fed Spirulina. J. Pharm. Belg. 43: 29–36.
Chamorro GA, Morelos E, Salazar S, Hong E, Salazar M (1995) Evaluation of the teratological potential of the new antihypertensive 5-methoxytryptamine, β-methylcarboxylate hydrochloride (indorenate), in mice. Arch. Med. Res. 26: 69–73.
Chamorro G, Salazar M (1990) Estudio teratogénico de Spirulina en ratón. Arch. Latinoam. Nutr. 86–90.
Chamorro G, Salazar M, Pages N (1996) Dominant lethal study of Spirulina maxima in male and female rats after short-term feeding. Phytother. Res. 10: 28–32.
Dillon JC, Phan PA. (1993) Spirulina as a source of proteins in human nutrition. Bull. Inst. Ocean. 12: 103–107.
Guideline on detection of toxicity to reproduction for medicinal products. 1993. ICH harmonised tripartite guideline. Endorsed by ICH Steering Committee, June 1993, Washington D.C.
IUPAC. International Union of Pure and Applied Chemistry. 1974. Proposed guidelines for testing of single cell protein destined as a major protein source for animal feed. Information Bulletin. Technical Report No. 12, IUPAC Secretariat. Oxford.
Krishnakumari MK, Ramesh HP, Venkataraman L (1982) Food safety evaluation: acute, oral and dermal effects of the algae Scenedesmus acutus and Spirulina platensis on albino rats. J. Food Protect. 44: 934–935.
Pabst W, Payer HD, Rolle I, Soeder C (1978) Safety evaluation of the microalga, Scenedesmus acutus. I. Biological and haematological data from multigeneration feeding studies in mice. Arch. Hydrobiol. Limnol. 11: 127–138.
PAG. Protein Advisory Group of the United Nations Systems (1974) PAG-Guidelines No. 6 for Clinical Testing of Novel Sources of Protein: PAG Bulletin 4. United Nations, New York, 17–31.
Salazar M, Chamorro GA, Salazar S, Steele CE (1996) Effect of Spirulina maxima consumption on reproduction and peri-and postnatal development in rats. Food Chem. Toxicol. 34: 353–359.
Santillán C (1982) Mass production of Spirulina. Experientia 38: 40–43.
Staples RE, Schnell VL (1964) Refinements in rapid clearing technique in the KOH-alizarin red S method for fetal bone. Stain Technol. 39: 61–63.
Steele CE, Copping GP (1990) Teratogen testing. In Coop AJ, Cockroft DL (eds), Postimplantation mammaliam embryos. A practical approach IRL Press at Oxford University Press, Oxford, 221–234.
Tranquille N, Emeis JJ, de Chambure D, Binot R, Tamponnet C (1994) Spirulina acceptability trials in rats. A study for the ‘Melissa’ life-support system. Adv. Space Res. 14: 1167–1170.
Tyl RW (1988) Developmental toxicity in toxicologic research. In Ballantyne B(ed.), Perspectives in Basic and Applied Toxicology. Wright, London, 206–241.
Wilson JC (1965) Embryological considerations in teratology. In Wilson JG, Warkany J (eds), Teratology: Principles and Techniques. The University of Chicago Press. Chicago, 251–261.
Yoshino Y, Hirai Y, Takahashi H, Yamamoto N, Yamazaki N (1980) The chronic intoxication test on Spirulina product fed to Wistarstrain rats. Jap. J. Nutr. 38: 221–225.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chamorro, G., Salazar, S., Favila-Castillo, L. et al. Reproductive and peri- and postnatal evaluation of Spirulina maxima in mice. Journal of Applied Phycology 9, 107–112 (1997). https://doi.org/10.1023/A:1007994500084
Issue Date:
DOI: https://doi.org/10.1023/A:1007994500084