Abstract
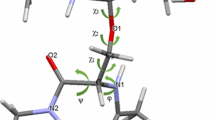
Synthetic oligosaccharide vaccines based on core STn (sialyl α2-6 GalNAc) carbohydrate epitopes are being evaluated by a number of biopharmaceutical firms as potential immunotherapeutics in the treatment of mucin-expressing adenocarcinomas. The STn carbohydrate epitopes exist as discontinuous clusters, O-linked to proximal serine and threonine residues within the mucin sequence. In an effort to probe the structure and dynamics of STn carbohydrate clusters as they may exist on the cancer-associated mucin, we have used NMR spectroscopy and MD simulations to study the effect of O-glycosylation of adjacent serine residues in a repeating (Ser)n sequence. Three model peptides/glyco-peptides were studied: a serine trimer containing no carbohydrate groups ((Ser)3 trimer); a serine trimer containing three Tn (GalNAc) carbohydrates α-linked to the hydroxyls of adjacent serine sidechains ((Ser.Tn)3 trimer); and a serine trimer containing three STn carbohydrates α-linked to the hydroxyls of adjacent serine sidechains ((Ser.STn)3 trimer). Our results demonstrate that clustering of carbohydrates shifts the conformational equilibrium of the underlying peptide backbone into a more extended and rigid state, an arrangement that could function to optimally present the clustered carbohydrate antigen to the immune system. Steric effects appear to drive these changes since an increase in the size of the attached carbohydrate (STn versus Tn) is accompanied by a stronger shift in the equilibrium toward the extended state. In addition, NMR evidence points to the formation of hydrogen bonds between the peptide backbone NH protons and the proximal GalNAc groups in the (Ser.Tn)3 and (Ser.STn)3 trimers. The putative peptide-sugar hydrogen bonds may also play a role in influencing the conformation of the underlying peptide backbone, as well as the orientation of the O-linked carbohydrate. The significance of these results will be discussed within the framework of developing clustered STn-based vaccines, capable of targeting the clustered STn epitopes on the cancer-associated mucin.
Similar content being viewed by others
References
Taylor-Papadimitriou J, Burchell J, Miles DW, Dalziel M, Biochim Biophys Acta 1455, 301–13 (1999).
Miles DW, Taylor-Papadimitriou J, Pharmacol Ther 82, 97–106 (1999).
Apostolopoulos V, Sandrin MS, McKenzie IF, J Mol Med 77, 427–36 (1999).
Apostolopoulos V, Pietersz GA, McKenzie IF, Curr Opin Mol Ther 1, 98–103 (1999).
van den Steen P, Rudd PM, Dwek RA, Podenakker G, Crit Rev Biochem Mol Biol 33, 151–208 (1998).
Rudd PM, Dwek RA, Crit Rev Biochem Mol Biol 32, 1–100 (1997).
Carlstedt I, Davies JR, Biochem Soc Trans 25, 214–9 (1997).
Koganty RR, Reddish MA, Longenecker BM. In Glycopeptides and Related Compounds: Synthesis, Analysis and Application, edited by Large DG, Warren CD (Dekker, New York, 1997), pp. 707–43.
Apostolopoulos V, McKenzie IF, Crit Rev Immunol 14, 293–309 (1994).
Girling A, Bartkova J, Burchell J, Gendler S, Gillett C, Taylor-Papadimitriou J, Int J Cancer 43, 1072–6 (1989).
Itzkowitz SH, Yuan M, Montgomery CK, Kjeldsen T, Takahashi HK, Bigbee WL, Kim YS, Cancer Res 49, 197–204 (1989).
Ogata S, Koganty R, Reddish M, Longenecker BM, Chen A, Perez C, Itzkowitz SH, Glycoconj J 15, 29–35 (1998).
Zhuang D, Yousefi S, Dennis JW, Cancer Biochem Biophys 12, 185–98 (1991).
Brockhausen I, Yang J, Dickinson N, Ogata S, Itzkowitz SH, Glycoconj J 15, 595–603 (1998).
Cao Y, Karstden U, Otto G, Bannascth P, Virchows Arch 434, 503–9 (1999).
Cao Y, Schlag PM, Karsten U, Virchows Arch 431, 159–66 (1997).
Springer GF, J Mol Med 75, 594–602 (1997).
Springer GF, Crit Rev Oncog 6, 57–85 (1995).
Springer GF, Science 224, 1198–1206 (1984).
Kishikawa T, Ghazizadeh M, Sasaki Y, Springer GF, Jpn J Cancer Res 90, 326–32 (1999).
Terasawa K, Furumoto H, Kamada M, Aono T, Cancer Res 56, 2229–32 (1996).
David L, Nesland JM, Clausen H, Carneiro F, Sobrinho-Simoes M, APMIS Suppl 27, 162–72 (1992).
Takao S, Uchikura K, Yonezawa S, Shinchi H, Aikou T, Cancer 86, 1966–75 (1999).
Imada T, Rino Y, Hatori S, Takahashi M, Amano T, Kondo J, Suda T, Hepatogastroenterology 46, 208–14 (1999).
Terashima S, Takano Y, Ohori T, Kanno T, Kimura T, Motoki R, Kawaguchi T, Surg Today 28, 682–6 (1998).
Werther JL, Tatematsu M, Klein R, Kurihara M, Kumagai K, Llorens P, Guidugli Neto J, Bodian C, Pertsemlidis D, Yamachika T, Kitou T, Itzkowitz S, Int J Cancer 69, 193–9 (1996).
Miles DW, Linehan J, Smith P, Filipe I, Br J Cancer 71, 1074–6 (1995).
Takahashi I, Maehara Y, Kusumoto T, Kohnoe S, Kakeji Y, Baba H, Sugimachi K, Br J Cancer 69, 163–6 (1994).
Kobayashi H, Terao T, Kawashima Y, J Clin Oncol 10, 95–101 (1992).
Soares R, Marinho A, Schmitt F, Pathol Res Pract 192, 1181–6 (1996).
Terasawa K, Furumoto H, Kamada M, Aono T, Cancer Res 56, 2229–32 (1996).
Bresalier RS, Ho SB, Schoeppner HL, Kim YS, Sleisenger MH, Brodt P, Byrd JC, Gastroenterology 110, 1354–67 (1996).
Ragupathi G, Howard L, Cappello S, Koganty RR, Qiu D, Longenecker BM, Reddish MA, Lloyd KO, Livingston PO, Cancer Immunol Immunother 48, 1–8 (1999).
MacLean GD, Miles DW, Rubens RD, Reddish MA, Longenecker BM, J Immunother Emphasis Tumor Immunol 19, 309–16 (1996).
Reddish MA, MacLean GD, Poppema S, Berg A, Longenecker BM, Cancer Immunol Immunother 42, 303–9 (1996).
Ogata S, Koganty R, Reddish M, Longenecker BM, Chen A, Perez C, Itzkowitz SH, Glycoconj J 15, 29–35 (1998).
Reddish MA, Jackson L, Koganty RR, Qiu D, Hong W, Longenecker BM, Glycoconj J 14, 549–60 (1997).
Zhang S, Walberg LA, Ogata S, Itzkowitz SH, Koganty RR, Reddish M, Gandhi SS, Longenecker BM, Lloyd KO, Livingston PO, Cancer Res, 55, 3364–8 (1995).
States DJ, Haberkorn RA, Ruben DJ, J Magn Reson 48, 286–92 (1982).
Piatini U, Sorenson OW, Ernst RR, J Am Chem Soc 104, 6800–1 (1982).
Rance M, Sorenson OW, Bodenhausen G, Wagner G, Ernst RR, Wüthrich K, Biochem Biophys Res Commun 117, 479–85 (1983).
Bax A, Davis DG, J Magn Reson 65, 355–60 (1985).
Kessler H, Griesinger C, Kerssebaum R, Wagner K, Ernst RR, J Am Chem Soc 109, 607–9 (1987).
Dauber-Osguthorpe P, Roberts VA, Osguthorpe DJ, Wolff J, Genest M, Hagler AT, Proteins 4, 31–47 (1988).
Balaji PV, Qasba PK, Rao VS, Glycobiology 4, 497–515 (1994).
Kozar T, Tvaroska I, Carver JP, Glyconjugate J 15, 187–91 (1998).
Gerken TA, Arch Biochem Biophys 247, 239–53 (1986).
Liu X, Sejbal J, Kotovych G, Koganty RR, Reddish MA, Jackson L, Gandhi SS, Mendonca AJ, Longenecker BM, Glyconjugate J 12, 607–17 (1995).
Mimura Y, Inoue Y, Maeji NJ, Chujo R, Int J Pept Prot Res 34, 363–8 (1989).
Butenhof KJ, Gerken TA, Biochemistry 32, 2650–63 (1993).
Wishart DS, Sykes BD, Richards FM, J Mol Biol 222, 311–33 (1991).
Osapay K, Case DA, J Biomol NMR 4, 215–30 (1994).
Wishart DS, Bigam, CG, Holm A, Hodges RS, Sykes BD, J Biol NMR 5, 67–81 (1995).
Dyson HJ, Wright PE, Annu Rev Biophys Chem 20, 519–38 (1991).
Live DH, Williams LJ, Kuduk SD, Schwarz JB, Glunz PW, Chen XT, Sames D, Kumar RA, Danishefsky SJ, Proc Natl Acad Sci USA 96, 3489–93 (1999).
Liang R, Adreotti AH, Kahne D, J Am Chem Soc 117, 10395–6 (1995).
Rose GD, Gierasch LM, Smith JA, Adv Protein Chem 37, 1–109 (1985).
Watts CR, Tessmer MR, Kallick DA, Lett Pept Sci 2, 59–70 (1995).
Andersen NH, Neidigh JW, Harris SM, Lee GM, Liu Z, Tong H, J Am Chem Soc 119, 8547–61 (1997).
Pardi A, Billeter M, Wüthrich K, J Mol Biol 180, 741–51 (1984).
Yao J, Feher VA, Espejo BF, Reymond MT, Wright PE, Dyson HJ, J Mol Biol 243, 736–53 (1994).
Schuster O, Klich G, Sinnwell V, Kranz H, Paulsen H, Mayer B, J Biomol NMR 14, 33–45 (1999).
Van den Steen P, Rudd PM, Dwek RA, Opdenakker G, Crit Rev Biochem Mol Biol 33, 151–208 (1998).
Bailey D, Renouf DV, Large DG, Warren CD, Hounsell EF, Carbohyd Res 324, 242–54 (2000).
Andreotti AH, Kahne D, J Am Chem Soc 115, 3352–3 (1993).
McManus AM, Otvos L, Hoffmann R, Craik DJ, Biochemistry 38, 705–14 (1999).
Gururaja TL, Ramasubbu N, Venugopalan P, Reddy MS, Ramalingam K, Levine MJ, Glycoconj J 15, 457–67 (1998).
Shogren R, Gerken TA, Jentoft N, Biochemistry 28, 5525–36 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schuman, J., Qiu, D., Koganty, R.R. et al. Glycosylations versus conformational preferences of cancer associated mucin core. Glycoconj J 17, 835–848 (2000). https://doi.org/10.1023/A:1010909011496
Issue Date:
DOI: https://doi.org/10.1023/A:1010909011496