Abstract
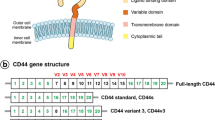
CD44, a hyaluronan (HA)3 receptor, belongs to a family of transmembrane glycoproteins which exists as several isoforms. Cell surface expression of certain CD44 isoforms is closely correlated with the progression and prognosis of breast cancers. A number of angiogenic factors (e.g., VEGF and FGF-2) and matrix degrading enzymes (MMPs) are tightly complexed with CD44 isoforms, suggesting that they are involved in the onset of oncogenic signals required for breast tumor cell invasion and migration. Most importantly, interaction of extracellular matrix components (e.g., HA) with cells triggers the cytoplasmic domain of CD44 isoforms to bind its unique downstream effectors (e.g., the cytoskeletal protein ankyrin or various oncogenic signaling molecules-Tiam1, RhoA-activated ROK, c-Src kinase and p185HER2) and to coordinate intracellular signaling pathways (e.g., Rho/Ras signaling and receptor-linked/non-receptor-linked tyrosine kinase pathways), leading to a concomitant onset of multiple cellular functions (e.g., tumor cell growth, migration and invasion) and breast tumor progression.
Similar content being viewed by others
REFERENCES
R. B. Dickson and M. E. Lippman (1996). New approaches in the therapy of breast cancer. Breast Cancer Res. & Treatment 38:1-2.
W. G. Jiang, M. C. A. Puntis, and M. B. Hallett (1994). Molecular and cellular basis of cancer invasion and metastasis: Implications for treatment. British J. Surgery 81:1576-1590.
D. A. Lauffenburger and A. F. Horwitz (1996). Cell migration: A physically integrated molecular process. Cell 84:359-369.
L. A. Goldstein, D. F. H. Zhou, L. J. Picker, C. N. Minty, R. F. Bargatze, J. F. Ding, and E. C. Butcher (1989). A human lymphocyte homing receptor, the hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell 56:1063-1072.
C. B. Underhill, S. J. Green, P. M. Comoglio, and G. Tarone (1987). The hyaluronate receptor is identical to a glycoprotein of 85,000 Mr. (gp85) as shown by a monoclonal antibody that interferes with binding activity. J. Biol. Chem. 262:13142-13146.
T. C. Laurent and J. R. Fraser (1992). Hyaluronan. FASEB J. 6:2397-2404.
S. J. Green, G. Tarone, and C. B. Underhill (1988). Aggregation of macrophages and fibroblasts is inhibited by a monoclonal antibody to the hyaluronate receptor. Exp. Cell. Res. 178:224-232.
D. C. West and S. Kumar (1989). The effect of hyaluronate and its oligosaccharides on endothelial cell proliferation and monolayer integrity. Exp. Cell. Res. 183:179-196.
E. A. Turley, L. Austen, K. Vandeligt, and C. Clary (1991). Hyaluronan and a cell-associated hyaluronan binding protein regulate the locomotion of ras-transformed cells. J. Cell. Biol. 112:1041-1047.
P. Rooney, S. Kumar, and M. Wang (1995). The role of hyaluronan in tumor neovascularization. Int J Cancer 60:632-636.
G. R. Screaton, M. V. Bell, D. G. Jackson, F. B. Cornelis, U. Gerth, and J. I. Bell (1992). Genomic structure of DNA coding the lymphocyte homing receptor CD44 reveals 12 alternatively spliced exons. Proc. Natl. Acad. Sci. U. S. A. 89:12160-12164.
V. B. Lokeshwar and L. Y. W. Bourguignon (1991). Posttranslational protein modification and expression of ankyrin binding site(s) in GP85(Pgp-1/CD44) and its biosynthetic precursors during T-lymphoma membrane biosynthesis. J. Biol. Chem. 266:17983-17989.
L. Y. W. Bourguignon, V. B. Lokeshwar, J. He, X. Chen, and G. J. Bourguignon (1992). ACD44-like endothelial transmembrane glycoprotein (GP116) Interacts with extracellular matrix and ankyrin. Mol. Cell. Biol. 12:4464-4471.
V. B. Lokeshwar, N. Iida, and L. Y. W. Bourguignon (1996). The cell adhesion molecule, GP116 is a new CD44 variant (ex14/v10) involved in hyaluronic acid binding and endothelial cell proliferation. J. Biol. Chem. 271:23853-23864.
P. Dall, K.-H. Heider, H.-P., Sinn, P. Skroch-Angel, G. Adolf, M. Kaufmann, P. Herrlich, and H. Ponta (1995). Comparison of immunohistochemistry and RT-PCR for detection of CD44v-expression, a new prognostic factor in human breast cancer. Int. J. Cancer. 60:471-477.
N. Iida and L. Y. W. Bourguignon (1995). New CD44 splice variants associated with human breast cancers. J. Cell. Physiol. 162:127-133.
M. Kaufmann, K. H. Heider, H. P. Sinn, G. von Minckwitz, H. Ponta, and P. Herrlich (1995). CD44 variant exon epitopes in primary breast cancer and length of survival. Lancet 345:615-619.
M. S. Sy, H. Mori, and D. Liu (1997). CD44 as a marker in human cancers. Curr. Opin. Oncol. 9:108-112.
E. Kalish, N. Iida, F. L. Moffat, and L. Y. W. Bourguignon (1999). A new CD44v3-containing isoform is involved in tumor cell migration and human breast cancer progression. Front Biosci. 4:1-8.
E. Horst, C. J. Meijer, T. Radaszkiewicz, G. J. Ossekoppele, J. H. Van Krieken, and S. T. Pals (1990). Adhesion molecules in the prognosis of diffuse large-cell lymphoma: Expression of a lymphocyte homing receptor (CD44), LFA (CD11a/18), and ICAM-1 (CD54). Leukemia 4:595-599.
S. Jalkanen, H. Joensuu, K. O. Soderstrom, and P. Klemi (1991). Lymphocyte homing and clinical behavior of non-Hodgkin's lymphoma. J. Clin. Invest. 87:1835-1840.
L. Y. W. Bourguignon, H. B. Zhu, A. Chu, L. Zhang, and Mien-Chie Hung (1997). Interaction between the adhesion receptor, CD44 and the oncogene product, p185HER2, pomotes human ovarian tumor cell activation. J. Biol. Chem. 272:27913-27918.
D. Zhu and L. Y. W. Bourguignon (1998). The ankyrin-binding domain of CD44s is involved in regulating hyaluronic acid-mediated function and prostate tumor cell transformation. Cell Motil. Cytoskel. 39:209-222.
L. Y. W. Bourguignon, H. Zhu, L. Shao, and Y. W. Chen (2001). CD44 interaction with c-Src kinase promotes cortactin-mediated cytoskeleton function and hyaluronic acid (HA)-dependent ovarian tumor cell migration. J. Biol. Chem. 276:7327-7336.
D. J. Slamon, G. Williams, L. A. Jones, J. A. Holt, S. G. Wong, D. E. Keith, W. J. Levin, S. G. Stuart, J. Udove, A. Ullrich, and M. F. Press (1989). Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244:707-710.
D. K. Luttrell, A. Lee, T. J. Lansing, R. M. Crosby, K. D. Jung, D. Willard, M. Luther, M. Rodriguez, J. Berman, and T. M. Gilmer (1994). Involvement of pp60c-Src with two major signaling pathways in human breast cancer. Proc. Natl. Acad. Sci. U.S.A. 91:83-87.
N. Rahimi, W. Hung, E. Tremblay, R. Saulnier, and B. Elliott (1998). c-Src kinase activity is required for hepatocyte growth factor-induced motility and anchorage-independent growth of mammary carcinoma cells. J. Biol. Chem. 273:33714-33721.
D. Naor, R. V. Sionov, and D. Ish-Shalom (1997). CD44: structure, function, and association with the malignant process. Adv. Cancer. Res. 71:241-319.
D. Liu and M. S. Sy (1996). A cysteine residue located in the transmembrane domain of CD44 is important in binding of CD44 to hylauronic acid. J. Exp. Med. 183:1987-1994.
L. Y. W. Bourguignon, E. L. Kalomiris, and V. B. Lokeshwar (1991). Acylation of the lymphoma transmembrane glycoprotein, GP85, may be required for GP85-ankyrin interaction. J. Biol. Chem. 266:11761-11765.
R. Karni, R. Jove, and A. Levitzki (1999). Inhibition of pp60c-Src reduces Bcl-XL expression and reverses the transformed phenotype of cells overexpressing EFG and HER-2 receptors. Oncogene 18:4654-4662.
I. Dikic, G. Tokiwa, S. Lev, S. A. Courtneidge, and J. Schlessinger (1996). A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature 383:547-550.
G. Koopman, K. H. Heider, E. Horst, G. R. Adolf, F. van den Berg, H. Ponta, and P. Herrlich (1993). Activated human lymphocytes and aggressive non-Hodgkin's lymphomas express a homologue of the rat metastasis-associated variant of CD44. J. Exp. Med. 177:897-904.
U. Gunthert, M. Hofmann, M, Rudy, S. Reber, M. Zoller, I. Haussmann, and S. Matzku (1991). A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 65:13-24.
K. Friedrichs, F. Franke, B.-W. Lisboa, G. Kugler, I. Gille, H.-J. Terpe, F. Holzel, H. Maass, and U. Gunthert (1995). CD44 isoforms correlate with cellular differentiation but not with prognosis in human breast cancer. Cancer Res. 55:5424-5433.
N. Iida and L. Y. W. Bourguignon (1997). Coexpression ofCD44 Variant (v10/ex 14) and CD44s in human mammary epithelial cells promotes tumorinogenesis. J. Cell. Physiol. 171:152-160.
I. Stamenkovic, M. Amiot, J. M. Pesando, and B. Seed (1991). The hemopoietic and epithelial forms of CD44 are distinct polypeptides with different adhesion potentials for hyalurononan-bearing cells. EMBO J. 10:343-347.
M. Bernfield, M. Gotte, P. W. Park, O. Reizes, M. L. Fitzgerald, J. Lincecum, and M. Zako (1999) Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68:729-777.
K. Bennett, D. G. Jackson, J. C. Simon, E. Tanczos, R. Peach, B. Modrell, I. Stamenkovic, G. Plowman, and A. Aruffo (1995). CD44 isoforms containing exon v3 are responsible for the presentation of heparin-binding growth factor. J. Cell. Biol. 128:687-698.
H. U. Grimme, C. C. Termeer, K. L. Bennett, J. M. Weiss, E. Schopf, A. Aruffo, and J. C. Simon (1999). Colocalization of basic fibroblast growth factor and CD44 isoforms containing the variable spliced exon v3 (CD44v3) in normal skin and in epidermal skin cancers. British. J. Dermatol. 141:824-832.
L. Y. W. Bourguignon, D. Zhu, and H. Zhu (1998). CD44 isoform-cytoskeleton interaction in oncogenic signaling and tumor progression. Front Biosci. 3:637-649.
H. F. Dvorak, L. F. Brown, M. Detmar, and A. M. Dvorak (1995). Review: Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 146:1029-1039.
A. T. W. Cheung, L. J. T. Young, P. C. Y. Chen, C. Y. Chao, A. Ndoye, P. A. Barry, W. J. Muller, and R. D. Cardiff (1997). Microcirculation and metastasis in a new mammary tumor model system. Int. J. Oncol. 11:69-77.
L. Y. W. Bourguignon, Z. Gunja-Smith, N. Iida, H. B. Zhu, L. J. T. Young, W. Muller, and R. D. Cardiff (1998) CD44v3,8–10-cytoskeleton interaction is involved in matrix matalloproteinase (MMP-9) function and tumor cell migration and invasion in metastatic breast tumor cells. J. Cell. Physiol. 176:206-215.
L. A. Liotta (1984). Tumor invasion and metastasis: Role of the basement membrane. Am. J. Pathol. 117:339-348.
W. T. Chen (1996). Proteases associated with invadopodia, and their role in degradation of extracellular matrix. Enzyme & Protein 49:59-71.
L. Y. W. Bourguignon (1996). Interaction between membrane-cytoskeleton and CD44 during lymphocyte signal transduction and cell adhesion. In W. J. Nelson (ed.), Current Topics in Membranes 43:293-312.
V. B. Lokeshwar, N. Fregien, and L. Y. W. Bourguignon (1994). Ankyrin binding domain of CD44(GP85) is required for the expression of hyaluronic acid-mediated adhesion function. J. Cell. Biol. 126:1099-1109.
V. Bennet (1992). Ankyrins. J. Biol. Chem. 267:8703-8706.
M. A. De Matteis and J. S. Morrow (1998). The role of ankyrin and spectrin in membrane transport and domain formation. Curr. Opin. Cell. Biol. 10:542-549.
L. L. Peters, K. M. John, F. M. Lu, E. M. Eicher, A. Higgins, M. Yialamas, L. C. Turtzo, A. J. Otsuka, and S. E. Lux (1995). Ank3 (epithelial ankyrin), a widely distributed new member of the ankyrin gene family and the major ankyrin in kidney, is expressed in alternatively spliced forms, including forms that lack the repeat domain. J. Cell. Biol. 130:313-330.
L. Davis and V. Bennett (1990). Mapping the binding sites of human erythrocyte ankyrin of the anion exchanger and spectrin. J. Biol. Chem. 265:10589-10596.
O. S. Platt, S. E. Lux, and J. F. Falcone (1993). A highly conserved region of human erythrocyte ankyrin contains the capacity to bind spectrin. J. Biol. Chem. 268:24421-24426.
D. Zhu and L. Y. W. Bourguignon (2000). Interaction between CD44 and the repeat domain of ankyrin promotes hyaluronic acid (HA)-mediated ovarian tumor cell migration. J. Cell. Physiol. 183:182-195.
L. Y. W. Bourguignon, H. Zhu, L. Shao, and Y. W. Chen (2000). Ankyrin-Tiam1 interaction promotes Rac1 signaling and metastatic breast tumor cell invasion and migration. J. Cell. Biol. 150:177-191.
L. Y. W. Bourguignon, H. B. Zhu, N. Iida, and L. Shao (1998). The cytoplasmic deletion (dominant-negative) mutant of CD44s down-regulates hyaluronan-mediated Tiam1-Rac1 signaling and IL-8 gene activation in human mammary epithelial cells. Mol. Biol. Cell. 9:301a.
A. Hall (1998). Rho GTPase and the actin cytoskeleton. Science 279:509-514.
L. Y. W. Bourguignon, H. Zhu, L. Shao, D. Zhu, and Y. W. Chen (1999). Rho-Kinase (ROK) promotes CD44v3,8–10-Ankyrin interaction and tumor cell migration in metastatic breast cancer cells. Cell. Motil. Cytoskel. 43:269-287.
M. Amano, M. Ito, K. Kimura, Y. Fukata, K. Chihara, T. Nakano, Y. Matsuura, and K. Kaibuchi (1996). Phosphorylation and activation of myosin by Rho-associated kinase (Rhokinase). J. Biol. Chem. 271:20246-20249.
N. Kimura, M. Ito, M. Amano, K. Chihara, Y. Fukata, M. Nakafuku, B. Yamamori, J. Feng, T. Nakano, K. Okawa, K. Iwamatsu, and K. Kaibuchi (1996). Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273:245-248.
M. Amano, K. Chihara, K. Kimura, Y. Fukata, N. Nakamura, Y. Matsuura, and K. Kaibuchi (1997). Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science 275:1308-1311.
A. J. Ridley, H. F. Paterson, C. L. Johnston, D. Diekman, and A. Hall (1992). The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70:401-410.
F. Michiels, G. G. M. Habets, J. C. Stan, R. A. van der Kammen, and J. G. Collard (1995). A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature 375:338-340.
G. G. M. Habets, E. H. M. Scholtes, D. Zuydgeest, R. A. van der Kammen, J. C. Stam, A. Berns, and J. G. Collard (1994). Identification of an invasion-inducing gene, Tiam-1, that encodes a protein with homology to GDP-GTP exchangers for Rho-like proteins. Cell 77:537-549.
G. G. M. Habets, R. A. van der Kammen, J. C. Stam, F. Michiels, and J. G. Collard (1995). Sequence of the human invasioninducing Tiam-1 gene, its conservation in evolution and its expression in tumor cell lines of different tissue origin. Oncogene 10:1371-1376.
L. Y. W. Bourguignon, H. Zhu, L. Shao, and Y. W. Chen (2000). CD44 interaction with Tiam1 promotes Rac1 signaling and hyaluronic acid (HA)-mediated breast tumor cell migration. J. Biol. Chem. 275:1829-1838.
S. Oliferenko, I. Kaverina, J. V. Small, and L. A. Huber (2000). Hyaluronic acid (HA) binding to CD44 activates Rac1 and induces lamellipodia outgrowth. J. Cell. Biol. 148:1159-1164.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bourguignon, L.Y.W. CD44-Mediated Oncogenic Signaling and Cytoskeleton Activation During Mammary Tumor Progression. J Mammary Gland Biol Neoplasia 6, 287–297 (2001). https://doi.org/10.1023/A:1011371523994
Issue Date:
DOI: https://doi.org/10.1023/A:1011371523994