Abstract
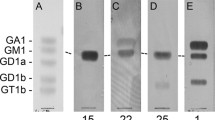
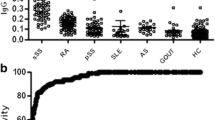
Galectin-3, a member of β-galactoside-binding lectins, is expressed and secreted by a variety of cell types including human intestinal epithelial cells. The presence of anti–galectin-3 antibody in the sera of patients was analyzed by immunoblotting using recombinant human galectin-3. A substantially higher percentage of sera from Crohn's disease patients contained anti-galectin-3 IgG autoantibodies than from patients with ulcerative colitis, primary biliary cirrhosis, or autoimmune hepatitis and of apparently healthy control volunteers. In Crohn's disease patients the titer of autoantibodies was high and interestingly correlated negatively with disease activity. To characterize and generate artificial epitopes (mimotopes), the anti-galectin-3 monoclonal antibodies A3A12 and B2C10 were used for biopannings of phage display nonapeptide libraries. These mimotopes interfered with the binding of autoantibodies to recombinant and native intestinal epithelial galectin-3. Our data may suggest that galectin-3 mimotopes could be used for the induction of IgG with desired specificity to regulate immune responses in Crohn's disease patients.
Similar content being viewed by others
REFERENCES
Barondes SH, Cooper DN, Gitt MA, Leffler H: Galectins. Structure and function of a large family of animal lectins. J Biol Chem 269:20807–20810, 1994
Liu F–T: Molecular biology of IgE–binding protein, IgE–binding factors, and IgE receptors. Crit Rev Immunol 10:289–306, 1990
Brassart D, Kolodziejczyk E, Granato D, Woltz A, Pavillard M, Perotti F, Frigeri LG, Liu F–T, Borel Y, Nesser JR: An intestinal galactose–specific lectin mediates the binding of murine IgE to mouse intestinal epithelial cells. Eur J Biochem 203:393–399, 1992
Huflejt ME, Jordan ET, Gitt MA, Barondes SH, Leffler H: Strikingly different localization of galectin–3 and galectin–4 in human colon adenocarcinoma T84 cells. Galectin–4 is localized at sites of cell adhesion. J Biol Chem 272:14294–14303, 1997
Seetharaman J, Kanigsberg A, Slaaby R, Leffler H, Barondes SH, Rini JM: X–ray crystal structure of the human galectin–3 carbohydrate recognition domain at 2.1–A resolution. J Biol Chem 273: 13047–13052, 1998
Hsu DK, Zuberi RI, Liu F–T: Biochemical and biophysical characterization of human recombinant IgE–binding protein, an S–type animal lectin. J Biol Chem 267:14167–14174, 1992
Liu F–T, Hsu DK, Zuberi RI, Hill PN, Shenhav A, Kuwabara I, Chen SS: Modulation of functional properties of galectin–3 by monoclonal antibodies binding to the non–lectin–domains. Biochemistry 35:6073–6079, 1996
Yang RY, Hill PN, Hsu DK, Liu F–T: Role of the carboxylterminal lectin domain in self–association of galectin–3. Biochemistry 37:4086–4092, 1998
Kuwabara I, Liu F–T: Galectin–3 promotes adhesion of human neutrophils to laminin. J Immunol 156:3939–3944, 1996
Frigeri LG, Zuberi RI, Liu F–T: εBP, a β–galactoside–binding animal lectin, recognizes IgE receptor (FcεRI) and activates mast cells. Biochemistry 32:7644–7649, 1993
Malchow H, Ewe K, Brandes JW, Goebell H, Ehms H, Sommer H, Jesdinsky H: European cooperative Crohn's disease study (ECCDS): Results of drug treatment. Gastroenterology 86:249–266, 1984
Best WR, Becktel JM, Singleton JW, Kern F Jr: Development of a Crohn's disease activity index. National cooperative Crohn's disease study. Gastroenterology 70:439–444, 1976
Fiocchi C: Inflammatory bowel disease: Etiology and pathogenesis. Gastroenterology 115:182–205, 1998
Ruemmele FM, Targan SR, Levy G, Dubinsky M, Braun J, Seidman EG: Diagnostic accuracy of serological assays in pediatric inflammatory bowel disease. Gastroenterology 115:822–829, 1998
Wesierska–Gadek J, Hohenauer H, Hitchman E, Penner E: Autoantibodies from patients with primary biliary cirrhosis preferentially react with the amino–terminal domain of nuclear pore complex glycoprotein gp120. J Exp Med 182:1159–1162, 1995
Wesierska–Gadek J, Grimm R, Hitchman E, Penner E: Members of the glutathione S–transferase gene family are antigens in autoimmune hepatitis. Gastroenterology 114:329–335, 1998
Felici F, Castagnoli L, Musacchio A, Jappelli R, Cesareni G: Selection of antibody ligands from a large library of oligopeptides expressed on a multivalent exposition vector. J Mol Biol 222:301–310, 1991
Luzzago A, Felici F, Tramontano A, Pessi A, Cortese R: Mimicking of discontinuous epitopes by phage–displayed peptides. I. Epitope mapping of human H ferritin using a phage library of constrained peptides. Gene 128:51–57, 1993
Jensen–Jarolim E, Leitner A, Kalchhauser H, Zürcher A, Ganglberger E, Bohle B, Scheiner O, Boltz–Nitulescu G, Breiteneder H: Peptide mimotopes displayed by phage inhibit antibody binding to Bet v 1, the major birch pollen allergen, and induced specific IgG in mice. FASEB J 12:1635–1642, 1998
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685, 1970
Towbin H, Staehelin T, Gordon J: Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354, 1979
Bresalier RS, Byrd JC, Wang L, Raz A: Colon cancer mucin: A new ligand for the β–galactoside–binding protein galectin–3. Cancer Res 56:4354–4357, 1996
Mathews KP, Konstantinov KN, Kuwabara I, Hill PN, Hsu DK, Zuraw BL, Liu F–T: Evidence for IgG autoantibodies to galectin–3, a β–galactoside–binding lectin (Mac–2, ε–binding protein, or carbohydrate binding protein 35) in human serum. J Clin Immunol 15:329–337, 1995
Suk K, Hwang DY, Lee MS: Natural autoantibody to galectin–9 in normal human sera. J Clin Immunol 19:158–165, 1999
Broberger O, Perlmann P: Autoantibodies in human ulcerative colitis. J Exp Med 110:657–674, 1959
Rutgeerts P, Vermeire S: Clinical value of the detection of antibodies in the serum for diagnosis and treatment of inflammatory bowel disease. Gastroenterology 115:1006–1009, 1998
Lutomski D, Joubert–Caron R, Lefebure C, Salama J, Belin C, Bladier D, Caron M: Anti–galectin–1 autoantibodies in serum of patients with neurological diseases. Clin Chim Acta 262:131–138, 1997
Tureci O, Schmitt H, Fadle N, Pfreundschuh M, Sahin U: Molecular definition of a novel human galectin which is immunogenic in patients with Hodgkin's disease. J Biol Chem 272:6416–6422, 1997
Abreu–Martin MT, Targan SR: Regulation of immune responses of the intestinal mucosa. Crit Rev Immunol 16:277–309, 1996
Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH: Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease. Clin Exp Immunol 102:448–455, 1995
Mey A, Leffler H, Hmama Z, Normier G, Revillard J–P: The animal lectin galectin–3 interacts with bacterial lipopolysaccharides via two independent sites. J Immunol 156:1572–1577, 1996
Ulevitch RJ, Tobias PS: Receptor–dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol 13:437–457, 1995
Strong SA, Pizarro TT, Klein JS, Cominelli F, Fiocchi C: Proin–flammatory cytokines differentially modulate their own expression in human intestinal mucosal mesenchymal cells. Gastroenterology 114:1244–1256, 1998
Jeng KC, Frigeri LG, Liu F–T: An endogenous lectin, galectin–3 (εBP/Mac–2), potentiates IL–1 production by human monocytes. Immunol Lett 42:113–116, 1994
Schreiber S, Nikolaus S, Hampe J, Hamling J, Koop I, Groessner B, Lochs H, Raedler A: Tumour necrosis factor–α and interleukin–1β in relapse of Crohn's disease. Lancet 353:459–461, 1999
Reinisch W, Gasche C, Tillinger W, Wyatt J, Lichtenberger C, Willheim M, Dejaco C, Waldhör T, Bakos S, Vogelsang H, Gangl A, Lochs L: Clinical relevance of serum interleukin–6 in Crohn's disease: Single point measurements, therapy monitoring, and prediction of clinical relapse. Am J Gastroenterol 94:2156–2164, 1999
Gasche C, Bakos S, Dejaco C, Tillinger W, Zakeri S, Reinisch W: IL–10 secretion and sensitivity in normal human intestine and inflammatory bowel disease. J Clin Immunol 20:362–370, 2000
Plevy SE, Landers CJ, Prehn J, Carramanzana NM, Deem RL, Shealy D, Targan SR: A role for TNF–_ and mucosal T helper–1 cytokines in the pathogenesis of Crohn's disease. J Immunol 159:6276–6282, 1997
Baert FJ, D'Haens GR, Peeters M, Hiele MI, Schaible TF, Shealy D, Geboes K, Rutgeerts PJ: Tumor necrosis factor alpha antibody (infliximab) therapy profoundly down–regulates the inflammation in Crohn's ileocolitis. Gastroenterology 116:22–28, 1999
Cohen RD, Tsang JF, Hanauer SB: Infliximab in Crohn's disease: First anniversary clinical experience. Am J Gastroenterol 95: 3469–3477, 2000
Nikoshkov A, Falorni A, Lajic S, Laureti S, Wedell A, Lernmark K, Luthman H: A conformation–dependent epitope in Addison's disease and other endocrinological autoimmune diseases maps to a carboxyl–terminal functional domain of human steroid 21–hydroxylase. J Immunol 162:2422–2426, 1999
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jensen-Jarolim, E., Neumann, C., Oberhuber, G. et al. Anti-Galectin-3 IgG Autoantibodies in Patients with Crohn's Disease Characterized by Means of Phage Display Peptide Libraries. J Clin Immunol 21, 348–356 (2001). https://doi.org/10.1023/A:1012240719801
Issue Date:
DOI: https://doi.org/10.1023/A:1012240719801