Abstract
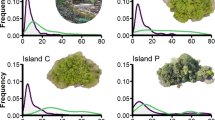
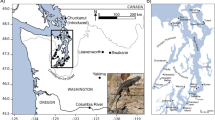
Populations of the lizards Anolis carolinensis and A. sagrei were experimentally introduced onto small islands in the Bahamas. Less than 15 years after introduction, we investigated whether the populations had diverged and, if so, whether differentiation was related to island vegetational characteristics or propagule size. No effect of founding population size was evident, but differentiation of A. sagrei appears to have been adaptive, a direct relationship existed between how vegetationally different an experimental island was from the source island and how much the experimental population on that island had diverged morphologically. Populations of A. carolinensis had also diverged, but were too few for quantitative comparisons. A parallel exists between the divergence of experimental populations of A. sagrei and the adaptive radiation of Anolis lizards in the Greater Antilles; in both cases, relative hindlimb length and perch diameter are strongly correlated. This differentiation could have resulted from genetic change or environmentally-driven phenotypic plasticity. Laboratory studies on A. sagrei from a population in Florida indicate that hindlimb length exhibits adaptive phenotypic plasticity. Further studies are required to determine if the observed differences among the experimental populations are the result of such plasticity. Regardless of whether the differences result from plasticity, genetic change, or both, the observation that anole populations differentiate rapidly and adaptively when exposed to novel environmental conditions has important implications for understanding the adaptive radiation of Caribbean anoles.
Similar content being viewed by others
References
Ashton, E.H. & S. Zuckerman, 1951. The influence of geographic isolation on the skull of the green monkey (Cercopithecus aethiops sabaeus): II. the cranial dimensions of the St. Kitts and the African green monkey. Proc. R. Soc. Lond. 138: 204–212.
Autumn, K., Y.A. Liang, S. T. Hsieh, W. Zeach, W.P. Chan, T.W. Kenny, R. Fearing & R.J. Full, 2000. Adhesive force of a single gecko foot-hair. Nature 405: 681–685.
Baker, A.J., 1980. Morphometric differentiation in New Zealand populations of the house sparrow (Passer domesticus). Evolution 34: 638–653.
Baker, A.J. & A. Moeed, 1979. Evolution in the introduced New Zealand populations of the common myna, Acridotheres tristis (Aves: Sturnidae). Can. J. Zool. 57: 570–584.
Barton, N.H., 1989. Founder effect speciation, pp. 229–256 in Speciation and its Consequences, edited by D. Otte & J.A. Endler. Sinauer Assoc., Sunderland, MA.
Barton, N.H., 1996. Natural selection and random genetic drift as causes of evolution on islands. Phil. Trans. Royal Soc. Lond. 351: 785–795.
Barton, N.H. & B. Charlesworth, 1984. Genetic revolutions, founder effects, and speciation. Ann. Rev. Ecol. Syst. 15: 133–164.
Bernays, E.A., 1986. Diet-induced head allometry among foliagechewing insects and its importance for graminivores. Science 231: 495–497.
Berry, R.J., 1964. The evolution of an island population of the house mouse. Evolution 18: 468–483.
Beuttell, K. & J.B. Losos, 1999. Ecological morphology of Caribbean anoles. Herpetol. Monogr. 13: 1–28.
Bookstein, F.L., 1989. 'size and shape': a comment on semantics. Syst. Zool. 38: 173–180.
Booth, F.W. & E.W. Gould, 1975. Effects of training and disuse on connective tissue, pp. 83–112 in Exercise and Sports Sciences Reviews, Vol. 3, edited by J.H. Wilmore & J.F. Keogh. Academic Press, New York.
Bryant, E.H. & L.M. Meffert, 1993. The effect of a serial founder-flush cycles on quantitative genetic variation in the housefly. Heredity 70: 122–129.
Bryant, E.H. & L.M. Meffert, 1996. Morphometric differentiation in serially bottlenecked populations of the housefly. Evolution 50: 935–940.
Burnaby, T.P., 1966. Growth-invariant discriminant functions and generalized distances. Biometrics 22: 96–110.
Buskirk, E.R., K.L. Andersen & J. Brozek, 1956. Unilateral activity and bone and muscle development in the forearm. Res. Quart. Amer. Assoc. Health Phys. Educ. 27: 127–131.
Carlquist, S., 1974. Island Biology. Columbia Univ. Press, New York.
Carroll, S.P. & C. Boyd, 1992. Host race radiation in the soapberry bug: natural history with the history. Evolution 46: 1052–1069.
Carson, H.L. & A.R. Templeton, 1984. Genetic revolutions in relation to speciation phenomena: the founding of new populations. Ann. Rev. Ecol. Syst. 15: 97–131.
Cheverud, J.M. & E.J. Routman, 1996. Epistasis as a source of increased additive genetic variance at population bottlenecks. Evolution 50: 1042–1051.
Cheverud, J.M., T.T. Vaughn, L.S. Pletscher, K. King-Ellison, J. Bailiff, E. Adams, C. Erickson & A. Bonislawski, 1999. Epistasis and the evolution of additive genetic variance in populations that pass through a bottleneck. Evolution 53: 1009–1018.
Coker, W.C., 1905. Vegetation of the Bahama Islands, pp. 185–270 in The Bahama Islands, edited by G.B. Shattuck. Macmillan, New York.
Conant, S., 1988. Geographic variation in the Laysan finch (Telespyza cantans). Evol. Ecol. 2: 270–282.
Erickson, G.M., 1997. The Evolution of the Biomechanical Attributes of Long Bones. Ph.D. Dissertation, University of California, Berkeley.
Fox, W., 1963. Special tubules for sperm storage in female lizards. Nature 198: 500–501.
Frankel, O.H. & M.E. Soulé, 1981. Conservation and Evolution. Cambridge Univ. Press, Cambridge.
Frankham, R., K. Lees, M.E. Montgomery, P.R. England, E.H. Lowe & D.A. Briscoe, 1999. Do population size bottlenecks reduce evolutionary potential? Anim. Cons. 2: 25–260.
Franklin, I.R., 1980. Evolutionary change in small populations, pp. 135–149 in Conservation Biology: An Evolutionary-Ecological Perspective, edited by M.E. Soulé & B.A. Wilcox. Sinauer Associates, Sunderland, Ma.
Gilpin, M.E. & M.E. Soulé, 1986. Minimum viable populations: processes of species extinction, pp. 19–34 in Conservation Biology: the Science of Scarcity and Diversity, edited byM.E. Soulé. Sinauer Associates, Sunderland, Ma.
Gingerich, P.D., 1983. Rates of evolution: effects of time and temporal scaling. Science222: 159–161.
Glossip, D. & J.B. Losos, 1997. Ecological correlates of number of subdigital lamellae in anoles. Herpetologica 53: 192–199.
Goodnight, C.J., 1987. On the effect of founder events on epistatic genetic variance. Evolution 41: 80–91.
Hendry, A.P. & M. T. Kinnison, 1999. The pace of modern life: measuring rates of contemporary microevolution. Evolution 53:1637–1653.
Huxley, J., 1942. Evolution: TheModern Synthesis. Harper &Bros., New York, 645 pp.
Irschick, D.J. & J.B. Losos, 1998. A comparative analysis of the ecological significance of maximal locomotor performance in Caribbean Anolis lizards. Evolution 52: 219–226.
Irschick, D.J. & J.B. Losos, 1999. Do lizards avoid habitats in which performance is submaximal?: The relationship between sprinting capabilities and structural habitat use in Caribbean anoles. Amer. Natural. 154: 293–305.
James, F.C., 1983. Environmental component of morphological differentiation in birds. Science 221: 184–187.
James, F.C., 1991. Complementary descriptive and experimental studies of clinal variation in birds. Amer. Zool. 31: 694–706.
Johnston, R., 1992. Evolution in the rock dove: skeletal morphology. Auk 109: 530–542.
Johnston, R.F. & R.K. Selander, 1971. Evolution in the house sparrow. II. Adaptive differentiation in North American populations. Evolution25: 1–28.
Jones, H.H., J.D. Priest, W.C. Hayes, C.C. Tichenor & D.A. Nagel, 1977. Humeral hypertrophy in response to exercise. J. Bone Joint Surg. 59–A: 204–208.
Kato, S. & T. Ishiko, 1966. Obstructed growth of children' bones due to excessive labor in remote corners, p. 479 in Proc. Int. Congress Sport Sci., edited by K. Kato. Japanese Union of Sport Sciences, Tokyo.
Kiiskinen, A., 1977. Physical training and connective tissues in young mice - physical properties of achilles tendons and long bones. Growth41: 123–137.
Kinnison, M.T. & A.P. Hendry, 2001. The pace of modern life. II: from rates to pattern and process. Genetica 112-113: 145–164.
Lints, F.A. & M. Bourgois, 1982. A test of the genetic revolution hypothesis of speciation, pp. 423–436 in Advances in Genetics, Development, and Evolution of Drosophila, edited by S. Lakovaara.Plenum Press, NY.
Loitz, B.J. & R.F. Zernicke, 1992. Strenuous exercise-induced remodelling of mature bone: relationships between in vivo strains and bone mechanics. J. Exp. Biol. 170: 1–18.
Losos, J.B., 1990a. The evolution of form and function: morphology and locomotor performance in West Indian Anolis lizards. Evolution 44: 1189–1203.
Losos, J.B., 1990b. Ecomorphology, performance capability, and scaling of West Indian Anolis lizards: an evolutionary analysis. Ecol. Mon. 60: 369–388.
Losos, J.B., D.A. Creer, D. Glossip, R. Goellner, A. Hampton, G. Roberts, N. Haskell, P. Taylor & J. Etling, 2000. Evolutionary implications of phenotypic plasticity in the Hindlimb of the lizard Anolis sagrei. Evolution 54: 301–305.
Losos, J.B. & D.J. Irschick. 1996. The effect of perch diameter on escape behaviour of Anolis lizards: laboratory-based predictions and field tests. Anim. Behav. 51: 593–602.
Losos, J.B., D.J. Irschick & T.W. Schoener, 1994. Adaptation and constraint in the evolution of specialization of Bahamian Anolis lizards. Evolution 48: 1786–1798.
Losos, J.B., T.R. Jackman, A. Larson, K. de Queiroz & L. Rodríguez-Schettino, 1998. Historical contingency and determinism in replicated adaptive radiations of island lizards. Science 279: 2115–2118.
Losos, J.B. & B. Sinervo, 1989. The effect of morphology and perch diameter on sprint performance of Anolis lizards. J. Exp. Biol. 145: 23–30.
Losos, J.B., K.I. Warheit & T.W. Schoener, 1997. Adaptive differentiation following experimental island colonization in Anolis lizards. Nature 387: 70–73.
Manly, B.F.J., 1991. Randomization and Monte Carlo Methods in Biology. Chapman & Hall, London.
Mayer, G.C., 1989. Deterministic patterns of community structure in West Indian reptiles and amphibians. Ph.D. Dissertation, Harvard University.
Mayr, E., 1963. Animal Species and Evolution. Belknap Press, Cambridge, MA.
Meyer, A. 1987. Phenotypic plasticity and heterochrony in Cichlasoma managuense (Pisces, Cichlidae) and their implications for speciation in cichlid fishes. Evolution 41: 1357–1369.
Nei, M., T. Maruyama & R. Chakraborty, 1975. The bottleneck effect and genetic variability in populations. Evolution 29: 1–10.
Neter, J., W. Wasserman & M.H. Kutner, 1985. Applied Linear Statistical Models: Regression, Analysis of Variance, and Experimental Design. R.D. Irwin,Homewood, Il., 2nd edn.
Patton, J.L. & P.V. Brylski, 1988. Pocket gophers in alfalfa fields: causes and consequences of habitat-related body size variation. Am. Nat. 130: 493–506.
Rand, A.S., 1964. Ecological distribution in anoline lizards of Puerto Rico. Ecology 45: 745–752.
Rand, A.S. & E. E. Williams, 1969. The anoles of La Palma. Breviora 327: 1–19.
Reznick, D.N. & Ghalamboor, 2001.
Reznick, D.N., F.H. Shaw, F.H. Rodd & R.G. Shaw, 1997. Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata). Science 275: 1934–1937.
Rhymer, J.M., 1992. An experimental study of geographic variation in avian growth and development. J. Evol. Biol. 5: 289–306.
Rice, W.R. & E.E. Hostert, 1993. Perspective: laboratory experiments on speciation: what have we learned in forty years? Evolution 47: 1637–1653.
Roughgarden, J.D. & E. Fuentes, 1977. The environmental determinants of size in solitary populations of West Indian Anolis lizards. Oikos 29: 44–51.
Rundle, H.D., A.O. Mooers & M.C. Whitlock, 1998. Single founder-flush events and the evolution of reproductive isolation. Evolution 52: 1850–1855.
Rundle, H.D., A.O. Mooers & M.C. Whitlock, 1999. Experimental tests of founder-flush: a reply to Templeton. Evolution 53: 1632–1633.
Schlichting, C.D. & M. Pigliucci, 1998. Phenotypic Evolution: A Reaction Norm Perspective. Sinauer Publ., Sinauer, MA.
Schmalhausen, I.I., 1949. Factors of Evolution. Blakiston, Philadelphia.
Schoener, T.W., 1968. The Anolis lizards of Bimini: resource partitioning in a complex fauna. Ecology49: 704–726.
Schoener, T.W., 1986. Patterns in terrestrial vertebrate versus arthropod communities: do systematic differences in regularity exist, pp. 556–586 in Community Ecology, edited by J. Diamond & T.J. Case. Harper & Row, New York.
Schoener, T.W. & A. Schoener, 1978. Inverse relation of survival of lizards with island size and avifaunal richness. Nature 274: 685–687.
Schoener, T.W. & A. Schoener, 1980. Densities, sex ratios, and population structure in four species of Bahamian Anolis lizards. J. Anim. Ecol. 49: 19–53.
Schoener, T.W. & A. Schoener, 1983. The time to extinction of a colonizing propagule of lizards increases with island area. Nature 302: 332–334.
Simpson, G.G., 1953. The Baldwin effect. Evolution 7: 110–117.
Sinervo, B. & J.B. Losos, 1991. Walking the tight rope: arboreal sprint performance among Sceloporus occidentalis lizard populations. Ecology 72: 1225–1233.
Sokal, R.R. & F.J. Rohlf, 1995. Biometry. W.H. Freeman & Co., New York.
Spiller, D.A., J.B. Losos & T.W. Schoener, 1998. Impact of a catastrophic hurricane on island populations. Science 281: 695–697.
St. Louis, V.L. & J.C. Barlow, 1991. Morphometric analyses of introduced and ancestral populations of the Eurasian tree sparrow. Wilson Bull. 103: 1–12.
Stamps, J.A., 1993. Sexual size dimorphism in species with asymptotic growth after maturity. Biol. J. Linn. Soc. 50: 123–145.
Stamps, J.A., V.V. Krishnan & R.M. Andrews, 1994. Analyses of sexual size dimorphism using null growth-based models.Copeia 1994: 598–613.
Steinhaus, A.H., 1933. Chronic effects of exercise. Physiol. Rev. 13: 103–147.
Sultan, S.E., 1987. Evolutionary implications of phenotypic plasticity in plants. Evol. Biol. 21: 127–178.
Templeton, A.R., 1980. The theory of speciation via the founder principle. Genetics94: 1011–1038.
Templeton, A.R., 1996. Experimental evidence for the genetictransilience model of speciation. Evolution 50: 909–915.
Templeton, A.R., 1999. Experimental tests of genetic transilience. Evolution 53: 1628–1632.
Trussell, G.C. & R.J. Etter, 2001. Integrating genetic and environmental forces that shape the evolution of geographic variation. Genetica.
Waddington, C.H., 1975. The Evolution of an Evolutionist. Cornell University Press, Ithaca.
West-Eberhard, M. J., 1989. Phenotypic plasticity and the origins of diversity. Ann. Rev. Ecol. Syst. 20: 249–278.
Williams, E.E., 1983. Ecomorphs, faunas, island size, and diverse end points in island radiations of Anolis, pp. 326–370 in Lizard Ecology: Studies of a Model Organism, edited by R.B. Huey, E.R. Pianka & T.W. Schoener. Harvard University Press, Cambridge.
Woo, S.L.-Y., S.C. Kuei, D. Amiel, M.A. Gomez, W.C. Hayes, F.C. White & W.H. Akeson, 1981. The effect of prolonged physical training on the properties of long bone: a study of Wolff' Law. J. Bone Joint Surg. 63–A: 780–786.
Wright, S., 1977. Evolution and the genetics of populations, in Experimental Results and Evolutionary Deductions, Vol. 3. University of Chicago Press, Chicago.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Losos, J.B., Schoener, T.W., Warheit, K.I. et al. Experimental studies of adaptive differentiation in Bahamian Anolis lizards. Genetica 112, 399–415 (2001). https://doi.org/10.1023/A:1013387705408
Issue Date:
DOI: https://doi.org/10.1023/A:1013387705408