Abstract
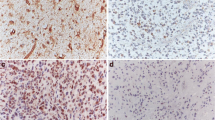
Astrocytic tumors are the most common human brain tumors. Establishment of tumor grade is a key determinant both in the choice of a therapeutic approach and in the prognosis. The diagnosis of astrocytic tumors is currently determined following histopathological analysis. The identification of molecular markers would offer a complementary tool for characterizing tumors with respect to their clinical behavior. In this study we determined the expression levels of 3 small GTP binding proteins (RhoA, RhoB and Rac1), of their inhibitor RhoGDI and of caveolin-1 in 24 human astrocytic tumors of grades I to IV. Our results demonstrated that the expression of RhoA and RhoB decreased significantly in all brain tumors studied and was inversely related with tumor of grade II to IV malignancy. The amount of caveolin-1 immunodetected was not significantly different from normal brain samples while the Rac1 expression level was diminished in astrocytic tumors of grades III and IV. Our finding that RhoA and RhoB expression levels are correlated to tumor malignancy suggests that they may serve as novel and efficient diagnostic markers for astrocytic brain tumors of histological grade II to IV and complement currently applied histopathological analysis.
Similar content being viewed by others
References
Stiles CD. Cancer of the central nervous system. Review of an AACR special conference in cancer research with the joint section on tumors of the AANS/CNS. Biochim Biophys Acta 1998; 1377: R1–10.
Pilkington GJ. The paradox of neoplastic glial cell invasion of the brain and apparent metastatic failure. Anticancer Res 1997; 17: 4103–5.
Mörk SJ, Wester K. Malignant gliomas: histological features, invasion, and relation to clinical symptoms. In Mikkelsen T, Bjerkvig R, Laerum OD et al. (eds): Brain Tumor Invasion: Biological, Clinical and Therapeutic Considerations. New York: Wiley-Liss 1998; 89–110.
Kleihues P, Burger PC, Scheithauer BW. Histological typing of tumors of the central nervous system. Ed. 2.World Health Organization. Berlin: Springer-Verlag 1993.
Pietsch T, Wiestler OD. Molecular neuropathology of astrocytic brain tumors. J Neurooncol 1997; 35: 211–22.
Greenberg HS, Chandler WF, Sandler HM. Brain Tumors. Contemporary Neurology Series. New York: Oxford University Press 1999; 350 pp.
Eibl RH, Pietsch T, Moll J et al. Expression of variant CD44 epitopes in human astrocytic brain tumors. J Neurooncol 1995; 26: 165–70.
Jaros E, Perry RH, Adam L et al. Prognostic implications of p53 protein, epidermal growth factor receptor, and Ki-67 labelling in brain tumours. Br J Cancer 1992; 66: 373–85.
Kachra Z, Beaulieu E, Delbecchi L et al. Expression of matrix metalloproteinases and their inhibitors in human brain tumors. Clin Exp Metastasis 1999; 17: 555–66.
Waha A, Baumann A, Wolf HK et al. Lack of prognostic relevance of alterations in the epidermal growth factor receptor-transforming growth factor-alpha pathway in human astrocytic gliomas. J Neurosurg 1996; 85: 634–41.
Banyard J, Anand-Apte B, Symons M et al. Motility and invasion are differentially modulated by Rho family GTPases. Oncogene 2000; 19: 580–91.
Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer 1999; 81: 682–7.
Sander EE, Collard JG. Rho-like GTPases: their role in epithelial cellcell adhesion and invasion. Eur J Cancer 1999; 35: 1302–8.
del Peso L, Hernandez-Alcoceba R, Embade N et al. Rho proteins induce metastatic properties in vivo. Oncogene 1997; 15: 3047–57.
Michiels F, Habets GG, Stam JC et al. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature 1995; 375: 338–40.
Stam JC, Michiels F, van der Kammen RA et al. Invasion of Tlymphoma cells: Cooperation between Rho family GTPases and lysophospholipid receptor signaling. EMBO J 1998; 17: 4066–74.
Chen Z, Sun J, Pradines A et al. Both farnesylated and geranylgeranylated RhoB inhibit malignant transformation and suppress human tumor growth in nude mice. J Biol Chem 2000; 275: 17974–8.
Gingras D, Gauthier F, Lamy S et al. Localization of RhoA GTPase to endothelial caveolae-enriched membrane domains. Biochem Biophys Res Commun1998; 247: 888–93.
Michaely PA, Mineo C, Ying YS et al. Polarized distribution of endogenous Rac1 and RhoA at the cell surface. J Biol Chem 1999; 274: 21430–6.
Smart EJ, Graf GA, McNiven MA et al. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol 1999; 19: 7289–304.
Galbiati F, Volonte D, Engelman JA et al. Targeted downregulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. EMBO J 1998; 17: 6633–48.
Suwa H, Ohshio G, Imamura T et al. Overexpression of the rhoC gene correlates with progression of ductal adenocarcinoma of the pancreas. Br J Cancer1998; 77: 147–52.
Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci USA 1995; 92: 1381–5.
Racine C, Belanger M, Hirabayashi H et al. Reduction of caveolin-1 gene expression in lung carcinoma cell lines. Biochem Biophys Res Commun 1999; 255: 580–6.
Bender FC, Reymond MA, Bron C et al. Caveolin-1 levels are down-regulated in human colon tumors, and ectopic expression of caveolin-1 in colon carcinoma cell lines reduces cell tumorigenicity. Cancer Res 2000; 60: 5870–8.
Lee SW, Reimer CL, Oh P et al. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene 1998; 16: 1391–7.
Lin R, Cerione RA, Manor D. Specific contributions of the small GTPases Rho, Rac, and Cdc42 to Dbl transformation. J Biol Chem 1999; 274: 23633–41.
Hall A. Rho GTPases and the actin cytoskeleton. Science 1998; 279: 509–14.
Scherer PE, Tang Z, Chun M et al. Caveolin isoforms differ in their N-terminal protein sequence and subcellular distribution. Identification and epitope mapping of an isoform-specific monoclonal antibody probe. J Biol Chem 1995; 270: 16395–401.
Ikezu T, Ueda H, Trapp BD et al. Affinity-purification and characterization of caveolins from the brain: Differential expression of caveolin-1,-2, and-3 in brain endothelial and astroglial cell types. Brain Res 1998; 804: 177–92.
Cleverley SC, Costello PS, Henning SW et al. Loss of Rho function in the thymus is accompanied by the development of thymic lymphoma. Oncogene 2000; 19: 13–20.
Michiels F, Collard JG. Rho-like GTPases: Their role in cell adhesion and invasion. Biochem Soc Symp 1999; 65: 125–46.
Zhang W, Razani B, Altschuler Y et al. Caveolin-1 inhibits epidermal growth factor-stimulated lamellipod extension and cell migration in metastatic mammary adenocarcinoma cells (MTLn3). Transformation suppressor effects of adenovirus-mediated gene delivery of caveolin-1. J Biol Chem 2000; 275: 20717–25.
Kleihues P, Soylemezoglu F, Schauble B et al. Histopathology, classi-fication, and grading of gliomas. Glia 1995; 15: 211–21.
Jung JM, Bruner JM, Ruan S et al. Increased levels of p21WAF1/Cip1 in human brain tumors. Oncogene 1993; 11: 2021–8.
Khalid H, Shibata S, Kishikawa M et al. Immunohistochemical analysis of progesterone receptor and Ki-67 labeling index in astrocytic tumors. Cancer 1997; 80: 2133–40.
Hiura T, Khalid H, Yamashita H et al. Immunohistochemical analysis of metallothionein in astrocytic tumors in relation to tumor grade, proliferative potential, and survival. Cancer 1998; 83: 2361–9.
Demeule M, Shedid D, Beaulieué et al. Expression of the multidrug resistance P-glycoprotein (MDR1) in human brain tumor. Intl J Cancer 2001; 93: 62-6.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Forget, MA., Desrosiers, R.R., Del Maestro, R.F. et al. The expression of Rho proteins decreases with human brain tumor progression: Potential tumor markers. Clin Exp Metastasis 19, 9–15 (2002). https://doi.org/10.1023/A:1013884426692
Issue Date:
DOI: https://doi.org/10.1023/A:1013884426692