Abstract
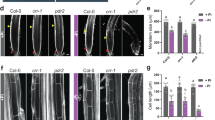
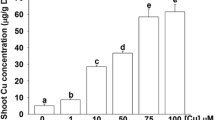
IRT1 and IRT2 are members of the Arabidopsis ZIP metal transporter family that are specifically induced by iron deprivation in roots and act as heterologous suppressors of yeast mutations inhibiting iron and zinc uptake. Although IRT1 and IRT2 are thought to perform redundant functions as root-specific metal transporters, insertional inactivation of the IRT1 gene alone results in typical symptoms of iron deficiency causing severe leaf chlorosis and lethality in soil. The irt1 mutation is characterized by specific developmental defects, including a drastic reduction of chloroplast thylakoid stacking into grana and lack of palisade parenchyma differentiation in leaves, reduced number of vascular bundles in stems, and irregular patterns of enlarged endodermal and cortex cells in roots. Pulse labeling with 59Fe through the root system shows that the irt1 mutation reduces iron accumulation in the shoots. Short-term labeling with 65Zn reveals no alteration in spatial distribution of zinc, but indicates a lower level of zinc accumulation. In comparison to wild-type, the irt1 mutant responds to iron and zinc deprivation by altered expression of certain zinc and iron transporter genes, which results in the activation of ZIP1 in shoots, reduction of ZIP2 transcript levels in roots, and enhanced expression of IRT2 in roots. These data support the conclusion that IRT1 is an essential metal transporter required for proper development and regulation of iron and zinc homeostasis in Arabidopsis.
Similar content being viewed by others
References
Askwith, C., Eide, D., Van Ho, A., Bernard, P.S., Li, L., Davis-Kaplan, S., Sipe, D.M. and Kaplan, J. 1994. The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell 76: 403–410.
Bechtold, N., Ellis, J. and Pelletier, G. 1993. In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci. Paris, Life Sciences, 316: 1194–1199.
Blaiseau, P.L., Lesuisse. E. and Camadro, J.M. 2001. Aft2p, a novel iron-regulated transcription activator that modulates, with Aft1p, intracellular iron use and resistance to oxidative stress in yeast. J. Biol. Chem. 276: 34221–34226.
Briat, J.-F., Fobis-Loisy, I., Grignon, N., Lobréaux. S., Pascal, N., Savino, G., Thoiron, S., von Wirén, N. and Van Wuytswinkel, O. 1995. Cellular and molecular aspects of iron metabolism in plants. Biol. Cell 84: 69–81.
Briat, J.-F. and Lobréaux, S. 1997. Iron transport and storage in plants. Trends Plant Sci. 2: 187–193.
Clough, S.J. and Bent, A.F. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743.
Cohen, C.K., Fox, T.C., Garvin, D.F. and Kochian, L.V. 1998. The role of iron-deficiency stress responses in stimulating heavymetal transport in plants. Plant Physiol. 116: 1063–1072.
Curie, C., Alonso, J.M., Le Jean, M., Ecker, J.R. and Briat, J.F. 2000. Involvement of NRAMP1 from Arabidopsis thaliana in iron transport. Biochem J. 347: 749–755.
Curie, C., Panaviene, Z., Loulergue, C., Dellaporta, S.L., Briat, J.-F. and Walker, E.L. 2001. Maize yellow stripe 1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 409: 346–349.
Dell'Orto, M., Santi, S., De Nisi, P., Cesco, S., Varanini, Z., Zocchi, G. and Pinton, R. 2000. Development of Fe-deficiency responses in cucumber (Cucumis sativus L.) roots: involvement of plasma membrane H+-ATPase activity. J. Exp. Bot. 51: 695–701.
Dix, D.R., Bridgham, J.T., Broderius, M.A., Byersdorfer, C.A. and Eide, D.J. 1994. The FET4 gene encodes the low affinity Fe(II) transport protein of Saccharomyces cerevisiae. J. Biol. Chem. 269: 26092–26099.
Eide, D., Broderius, M., Fett, J. and Guerinot, M.L. 1996. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc. Natl. Acad. Sci. USA 93: 5624.5628.
Grotz, N., Fox, T., Connolly, E., Park, W., Guerinot, M.L. and Eide, D. 1998. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc. Natl. Acad. Sci. USA 95: 7220–7224.
Guerinot, M.L. 2000. The ZIP family of metal transporters. Biochem. Biophys. Acta 1465: 190–198.
Koncz, C., Martini, N., Mayerhofer, N., Koncz-Kálmán, Z., Körber, H., Rédei, G.P. and Schell, J. 1989. High-frequency T-DNAmediated gene tagging in plants. Proc. Natl. Acad. Sci. USA 86: 8467–8471.
Koncz, C., Mayerhofer, R., Koncz-Kálmán, Z., Nawrath, C., Reiss, B., Rédei, G.P. and Schell, J. 1990. Isolation of a gene encoding a novel chloroplast protein by T-DNA tagging in Arabidopsis thaliana. EMBO J. 9: 1337–1346.
Koncz, C., Chua, N.-H. and Schell, J. 1992. Methods in Arabidopsis Research. World Scientific, Singapore
Koncz, C., Martini, N., Szabados, L., Hrouda, M., Bachmair, A. and Schell, J. 1994. Specialized vectors for gene tagging and expression studies. In Gelvin, S.B. and Schilperoort, R.A. (Eds) Plant Molecular Biology Manual, Kluwer Academic Press, Dordrecht, B2: pp. 1–22.
Korshunova, Y.O., Eide, D. Clark, G.W., Guerinot, M.L. and Pakrasi, H.B. 1999. The IRT1 protein from Arabidopsis thaliana is a metal transporter with a broad substrate specificity. Plant Mol. Biol. 40: 37–44.
Krysan, P.J., Young, J.C. and Sussman, M.R. (1999) T-DNA as an insertional mutagen in Arabidopsis. Plant Cell 12: 2283–2290.
Landsberg, E.C. 1994. Transfer cell formation in sugar beet roots induced by latent Fe deficiency. Plant Soil 165: 197–205.
Landsberg, E.C. 1996. Hormonal regulation of iron-stress response in sunflower roots: a morphological and cytological investigation. Protoplasma 194: 60–80.
Lyons, T.J., Gasch, A.P., Gaither, A.L., Botstein, D., Brown, P.O. and Eide, D.J. 2000. Genome-wide characterization of the Zap1p zinc-responsive regulon in yeast. Proc. Natl. Acad. Sci. USA 97: 7957–7962.
Mäser, P., Thomine, S., Schroeder, J.I., Ward, J.M., Hirschi, K., Sze, H., Talke, I.N., Amtmann, A., Maathuis, F.J.M., Sanders, D., Harper, J.F., Tchieu, J., Gribskov, M., Persans, M.W., Salt, D.E., Kim, S.A. and Guerinot, M.L. 2001. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 126: 1646–1667.
Mori, S. 1999. Iron acquisition in plants. Curr. Opin. Plant Biol. 2: 250–253.
Németh, K., Salchert, K., Putnoky, P., Bhalerao, R., Koncz-Kálmán, Z., Stankovic-Stangeland, B., Bakó, L., Mathur, J., Ökrész, L., Stabel, S., Geigenberger, P., Stitt, M., Rédei, G.P., Schell, J. and Koncz, C. 1998. Control of glucose and hormone responses by PRL1, a nuclear WD-protein, in Arabidopsis. Genes Devel. 12: 3059–3073.
Reynolds, E.S. 1963. The use of lead citrate at high pH as an electronopaque stain in electron microscopy. J. Cell. Biol. 17: 208–212.
Robinson, N.J., Procter, C.M., Connolly, E.L. and Guerinot, M.L. 1999. A ferric-chelate reductase for iron uptake from soils. Nature 397: 694–697.
Rogers, S.O. and Bendich, A.J., 1985. Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol. Biol. 5: 69–76.
Rogers, E.E., Eide, D.J. and Guerinot, M.L. 2000. Altered selectivity in an Arabidopsis metal transporter. Proc. Natl. Acad. Sci. USA 97: 12356–12360.
Sambrook, J., Fritsch, E.F. and Maniatis, T. 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Schmidt, W. and Bartels, M. 1996. Formation of root epidermal transfer cells in Plantago. Plant Physiol. 110: 217–225.
Schmidt, W., Tittel, J. and Schikora, A. 2000. Role of hormones in the induction of iron deficiency responses in Arabidopsis roots. Plant Physiol. 122: 1109–1118.
Shojima, S., Nishizawa, N.K., Fushiya, S., Nozoe, S., Irifune, T. and Mori, S. 1990. Biosynthesis of phytosiderophores. In vitro biosynthesis of 2'-deoxymugineic acid from L-methionine and nicotianamine. Plant Physiol. 93: 1497–1503.
Spurr, A.R. 1969. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruc. Res. 26: 31–43.
Takahashi, M., Nakanishi, H., Kawasaki, S., Nishizawa, N.K. and Mori, S. 2001. Enhanced tolerance of rice to low iron availability in alkaline soils using barley nicotianamine aminotransferase genes. Nature Biotech. 19: 466–469.
Thimm, O., Essigmann, B., Kloska, S., Altmann, T. and Buckhout, T.J. 2001. Response of Arabidopsis to iron deficiency stress as revealed by microarray analysis. Plant Physiol. 127: 1030–1043.
Thomine, S., Wang, R., Ward, J.M., Crawford, N.M. and Schroeder, J.I. 2000. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc. Natl. Acad. Sci. USA 97: 4991–4996.
Vert, G., Briat, J.-F. and Curie, C. 2001. Arabidopsis IRT2 gene encodes a root-periphery iron transporter. Plant J. 26: 181–189.
Von Wirén, N., Klair, S., Bansal, S., Briat, J.-F., Khodr, H., Shioiri, T., Leigh, R.A. and Hider, R.C. 1999. Nicotianamine chelates both Fe III and Fe II. Implications for metal transport in plants. Plant Physiol. 119: 1107–1114.
Waldo, G.S., Wright, E., Whang, Z.H., Briat, J.F., Theil, E.C. and Sayers, D.E. 1995. Formation of the ferritin iron mineral occurs in plastids. Plant Physiol. 109: 797–802.
Yi, Y. and Guerinot, M.L. 1996. Genetic evidence that induction of root Fe(III) chelate reductase activity is necessary for iron uptake under iron deficiency. Plant J. 10: 835–844.
Young, J.C., Krysan, P.J. and Sussman, M.R. 2001. Efficient screening of Arabidopsis T-DNA insertion lines using degenerate primers. Plant Physiol. 125: 513–518.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Henriques, R., Jásik, J., Klein, M. et al. Knock-out of Arabidopsis metal transporter gene IRT1 results in iron deficiency accompanied by cell differentiation defects. Plant Mol Biol 50, 587–597 (2002). https://doi.org/10.1023/A:1019942200164
Issue Date:
DOI: https://doi.org/10.1023/A:1019942200164