Abstract
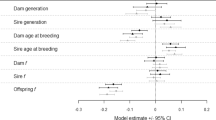
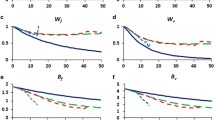
Many species require captive breeding to ensuretheir survival. The eventual aim of suchprograms is usually to reintroduce the speciesinto the wild. Populations in captivitydeteriorate due to inbreeding depression, lossof genetic diversity, accumulation of newdeleterious mutations and genetic adaptationsto captivity that are deleterious in the wild.However, there is little evidence on themagnitude of these problems. We evaluatedchanges in reproductive fitness in populationsof Drosophila maintained under benigncaptive conditions for 50 generations witheffective population sizes of 500 (2replicates), 250 (3), 100 (4), 50 (6) and 25(8). At generation 50, fitness in the benigncaptive conditions was reduced in smallpopulations due to inbreeding depression andincreased in some of the large populations dueto modest genetic adaptation. When thepopulations were moved to `wild' conditions,all 23 populations showed a marked decline(64–86%percnt;) in reproductive fitness compared tocontrols. Reproductive fitness showed acurvilinear relationship with population size,the largest and smallest population sizetreatments being the worst. Genetic analysesindicated that inbreeding depression andgenetic adaptation were responsible for thegenetic deterioration in `wild' fitness.Consequently, genetic deterioration incaptivity is likely to be a major problem whenlong-term captive bred populations ofendangered species are returned to the wild. Aregime involving fragmentation of captivepopulations of endangered species is suggestedto minimize the problems.
Similar content being viewed by others
References
Allendorf, FW (1993) Delay of adaptation to captive breeding by equalizing family size. Conserv. Biol., 7, 416–419.
Arnold SJ (1995) Monitoring quantitative genetic variation and evolution in captive populations. In: Population Management for Survival & Recovery: Analytical Methods and Strategies in Small Population Conservation (eds. Ballou JD, Gilpin M, Foose TJ), pp. 295–317. Columbia University Press, New York.
Ballou J, Lacy RC (1995) Identifying genetically important individuals for management of genetic diversity in pedigreed populations. In: Population Management for Survival and Recovery: Analytical Methods and Strategies in Small Population Conservation (eds. Ballou J, Gilpin M, Foose T), pp. 76–111. Columbia University Press, New York.
Beck BB, Rapaport LG, Stanley Price MR, Wilson AC (1994) Reintroduction of captive-born animals. In: Creative Conservation: Interactive Management of Wild and Captive Animals (eds. Olney PJS Mace GM, Feistner ATC), pp. 265–286. Chapman & Hall, London.
Borlase SC, Loebel DA, Frankham R, Nurthen RK, Briscoe DA, Daggard GE (1993) Modeling problems in conservation genetics using captive Drosophila populations: Consequences of equalization of family sizes. Conserv. Biol., 7, 122–131.
Bush GL, Neck RW, Kitto GB (1976) Screwworm eradication: Inadvertent selection for noncompetitive ecotypes during mass rearing. Science, 193, 491–493.
Caballero A, Toro MA, Lopez-Fanjul C (1991) The response to artificial selection from new mutations in Drosophila melanogaster. Genetics, 128, 89–102.
Chilcote MW, Leider SA, Loch JJ (1986) Differential reproductive success of hatchery and wild summer-run steelhead under natural conditions. Trans. Amer. Fisheries Soc., 115, 727–735.
Crnokrak P, Roff D (1999) Inbreeding depression in the wild. Heredity, 83, 260–270.
Cruden D (1949) The computation of inbreeding coefficients in closed populations. J. Hered., 40, 248–251.
Falconer DS, Mackay TFC (1996) Introduction to Quantitative Genetics (Fourth ed.). Harlow, Longman, Harlow, Essex.
Fleming IA, Gross MT (1993) Breeding success of hatchery and wild coho salmon (Oncorhynchus kisutch) in competition. Ecol. Applic., 3, 230–245.
Fleming IA, Hindar K, Mjolnerod IB, Jonsson B, Balstad T, Lamberg A (2000) Lifetime success and interactions of farm salmon invading a native population. Proc. Roy. Soc. Lond. B, 267, 1517–1523.
Frankham R (1995) Genetic management of captive populations for reintroduction. In: Reintroduction Biology of Australian and New Zealand Fauna (ed. Serena M), pp. 31–34. Surrey Beatty & Sons, Chipping Norton, NSW
Frankham R, Loebel DA (1992) Modeling problems in conservation genetics using captive Drosophila populations: Rapid genetic adaptation to captivity. Zoo Biol., 11, 333–342.
Frankham R, Manning H, Margan SH, Briscoe DA (2000) Does equalisation of family sizes reduce genetic adaptation to captivity? Anim. Conserv., 3, 357–363.
Frankham R, Yoo BH, Sheldon BL (1988) Reproductive fitness and artificial selection in animal breeding: Culling on fitness prevents a decline in reproductive fitness in lines of Drosophila melanogaster selected for increased inebriation time. Theor. Appl. Genet., 76, 909–914.
Fraser JM (1981) Comparative survival and growth of planted wild, hybrid, and domestic strains of brook trout (Salvelinus fontinalis) in Ontario Lakes. Can. J. Fish. Aquatic Sci., 38, 1372–1384.
Gilligan DM (2001) Conservation Genetics and Long Term Survival: Testing Models using Drosophila. Ph.D. thesis, Macquarie University, Sydney, Australia.
Gilligan DM, Woodworth LM, Montgomery ME, Briscoe DA, Frankham R (1997) Is mutation accumulation a threat to the survival of endangered populations? Conserv. Biol., 11, 1235–1241.
Gipps JHW (ed.) (1991) Beyond Captive Breeding: Re-introducing Endangered Mammals into the Wild. Clarendon Press, Oxford.
Haymer DS, Hartl DL (1982) The experimental assessment of fitness in Drosophila. I. Comparative measures of competitive reproductive success. Genetics, 102, 455–466.
Haymer DS, Hartl DL (1983) The experimental assessment of fitness in Drosophila. II. Comparison of competitive and noncompetitive measures. Genetics, 104, 343–352.
Heschel MS, Paige KN (1995) Inbreeding depression, environmental stress, and population size variation in scarlet gilia (Ipomopsis aggregata). Conserv. Biol., 9, 126–133.
Hill WG (1982) Predictions of response to artificial selection from new mutations. Genet. Res., 40, 255–278.
Hindar K, Ryman N, Utter F (1991) Genetic effects of cultured fish on natural fish populations. Can. J. Fish. Aquatic Sci., 48, 945–957.
Hoffmann AA, Parsons PA (1991) Evolutionary Genetics and Environmental Stress. Oxford University Press, Oxford.
Holland B, Rice WR (1999) Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc. Nat. Acad. Sci. USA, 96, 5083–5088.
IUCN (1987) IUCN Policy Statement on Translocation of Living Organisms: Introductions, Reintroductions and Restocking. IUCN Council, Gland, Switzerland.
IUCN (1996) 1996 IUCN Red List of Threatened Animals. IUCN Council, Gland, Switzerland.
Jungen H, Hartl DL (1979) Average fitness of populations of Drosophila melanogaster as estimated using compound-autosome strains. Evolution, 33, 359–370.
Kimura M (1983) The Neutral Theory of Molecular Evolution. Cambridge University Press, Cambridge, England.
Kimura M, Crow JF (1963) On the maximum avoidance of inbreeding. Genet. Res., 4, 399–415.
Lachance S, Magnan P (1990) Performance of domestic, hybrid, and wild strains of brook trout, Salvelinus fontinalis, after stocking: The impact of intra-and interspecific competition. Can. J. Fish. Aquatic Sci., 47, 2278–2284.
Lacy RC (1997) Importance of genetic variation in the viability of mammalian populations. J. Mammal., 78, 320–335.
Lande R (1995) Mutation and conservation. Conserv. Biol., 9, 782–791.
Latter BDH, Mulley JC (1995) Genetic adaptation to captivity and inbreeding depression in small laboratory populations of Drosophila melanogaster. Genetics, 139, 255–266.
Leary RF, Allendorf FW, Knudsen KL (1993) Null allele heterozygosity at two lactate dehydrogenase loci in rainbow trout are associated with decreased developmental stability. Genetica, 89, 3–13.
Leopold AS (1944) The nature of heritable wildness in turkeys. Condor, 46, 133–197.
Lynch M, Conery J, Burger R (1995) Mutational meltdowns in sexual populations. Evolution, 49, 1067–1080.
Lynch M, Walsh B (1998) Genetics and Analysis of Quantitative Traits. Sinauer, Sunderland, MA.
Margan SH, Nurthen RK, Montgomery ME, Woodworth LM, Briscoe DA Frankham R (1998) Single large or several small? Population fragmentation in the captive management of endangered species. Zoo Biol., 17, 467–480.
Miller PS (1994) Is inbreeding depression more severe in a stressful environment? Zoo Biol., 13, 195–208.
Montgomery ME, Ballou JD, Nurthen RK, Briscoe DA, Frankham R (1997) Minimizing kinship in captive breeding programs. Zoo Biol., 16, 377–389.
Montgomery ME, Woodworth LM, Nurthen RK, Gilligan DM, Briscoe DA Frankham R (2000) Relationships between population size and loss of genetic diversity: Comparisons of experimental results with theoretical predictions. Conserv. Genet., 1, 33–43.
Myers D, Sabath MD (1980) Genetic and phenotypic variability, genetic variance, and the success of establishment of insect introductions for the biological control of weeds. Proc. V Int. Sym. on Biological Control of Weeds, pp. 91–102. Brisbane, Australia.
Nunney L (2001) Managing captive populations for release: A population genetic perspective. In: Quality Control of Natural Enemies used in Biological Pest Control: Theoretical Background and Development of Testing Procedures (ed. van Lenteren JC). Cambridge University Press, Cambridge, UK.
Ralls K, Ballou JD (1983) Extinction: Lessons from zoos. In: Genetics and Conservation: A Reference for Managing Wild Animal and Plant Populations (eds. Schonewald-Cox CM, Chambers SM, MacBryde B, Thomas L), pp. 164–184. Benjamin/ Cummings, Menlo Park, CA.
Ralls K, Ballou J (eds.) (1986) Proceedings of the workshop on genetic management of captive populations. Zoo Biol., 5, 81–238.
Reisenbichler RR, Rubin SP (1999) Genetic changes from artificial propagation of Pacific salmon affect the productivity and viability of supplemented populations. ICES J. Mar. Sci., 56, 459–466.
Robertson A (1960) A theory of limits in artificial selection. Proc. Roy. Soc. Lond. B, 153, 234–249.
Robertson A (1964) The effect of non-random mating within inbred lines on the rate of inbreeding. Genet. Res., 5, 164–167.
Rumball W, Franklin IR, Frankham R, Sheldon BL (1994) Decline in heterozygosity under full-sib and double first-cousin inbreeding in Drosophila melanogaster. Genetics, 136, 1039–1049.
Shabalina SA, Yampolsky LY, Kondrashov AS (1997) Rapid decline in fitness in panmictic populations of Drosophila melanogaster maintained under relaxed natural selection. Proc. Nat. Acad. Sci. USA, 94, 13034–13039.
Soulé ME, Gilpin M, Conway W, Foose T (1986) The millennium ark: How long a voyage, how many staterooms, how many passengers? Zoo Biol., 5, 101–113.
Stanley Price M, Gordon I (1989) How to go wild. New Scient., 124(1688), 40–42.
Tudge C (1995) Captive audiences for future conservation. New Scient., 145(1962), 51–52.
Weber KE, Diggins LT (1990) Increased selection response in larger populations. II. Selection for ethanol vapor resistance in Drosophila melanogaster, at two population sizes. Genetics, 125, 585–597.
Wilson AC, Stanley Price MR (1994) Reintroduction as a reason for captive breeding. In: Creative Conservation: Interactive Management of Wild and Captive Animals (eds. Olney PJS, Mace GM, Feistner ATC), pp. 243–264. Chapman & Hall, London.
Woodworth LM (1996) Population Size in Captive Breeding Programs. Ph.D. thesis, Macquarie University, Sydney, Australia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woodworth, L.M., Montgomery, M.E., Briscoe, D.A. et al. Rapid genetic deterioration in captive populations: Causes and conservation implications. Conservation Genetics 3, 277–288 (2002). https://doi.org/10.1023/A:1019954801089
Issue Date:
DOI: https://doi.org/10.1023/A:1019954801089