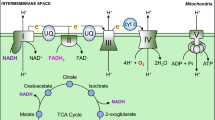
Abstract
Mammalian cells have multiple responses to low or zero oxygen concentrations. In the complete absence of oxygen, cells undergo cell death through apoptosis, and not necrosis. Apoptotic signaling during oxygen deprivation occurs through the release of cytochrome c and apaf-1 mediated caspase-9 activation. The upstream regulators of cytochrome c release are the Bcl-2 family members. Pro-apoptotic Bcl-2 family members such as bax or bak are clearly required to initiate cytochrome c/apaf-1/caspase-9 mediated cell death during oxygen deprivation. Here we review what is currently known oxygen deprivation induced cell death and speculate about initiating mechanisms resulting in the activation of pro-apoptotic Bcl-2 family members.
Similar content being viewed by others
References
Saikumar P, Dong Z, Weinberg JM, Venkatachalam MA Mechanisms of cell death in hypoxia/reoxygenation injury. Oncogene 1998; 17: 3341–3349.
Kang PM, Izumo S Apoptosis and heart failure: A critical review of the literature. Circ Res 2000; 86: 1107–1113.
Zipfel GJ, Lee JM, Choi DW Reducing calcium overload in the ischemic brain. N Engl J Med 1999; 341: 1543–1544.
Jain RK, Munn LL, Fukumura D Dissecting tumour pathophysiology using intravital microscopy. Nature Rev Cancer 2002; 2: 266–276.
Vaux DL, Korsmeyer SJ Cell death in development. Cell 1999; 96: 245–254.
Ameisen JC The origin of programmed cell death. Science 1996; 272: 1278–1279.
Evan G, Littlewood T A matter of life and cell death. Science 1998; 281: 1317–1322.
Kerr JF, Wyllie AH, Currie AR Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972; 26: 239–257.
Thornberry NA, Lazebnik Y Caspases: Enemies within. Science 1998; 281: 1312–1316.
Salvesen GS, Dixit VM Caspases: Intracellular signaling by proteolysis. Cell 1997; 91: 443–446.
Nicotera P, Leist M, Ferrando-May E Intracellular ATP: A switch in the decision between apoptosis and necrosis. Toxicol Lett 1998; 102–103: 139–142.
Savill J, Fadok V Corpse clearance defines the meaning of cell death. Nature 2000; 407: 784–788.
Thompson CB Apoptosis in the pathogenesis and treatment of disease. Science 1995; 267: 1456–1462.
Strasser A, Harris AW, Huang DC, Krammer PH, Cory S Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. Embo J 1995; 14: 6136–6147.
Green DR Apoptotic pathways: Paper wraps stone blunts scissors. Cell 2000; 102: 1–4.
Vander Heiden MG, Thompson CB Bcl-2 proteins: Regulators of apoptosis or of mitochondrial homeostasis? Nat Cell Biol 1999; 1: E209–216.
Huang DC, Strasser A BH3-Only proteins-essential initiators of apoptotic cell death. Cell 2000; 103: 839–842.
Wang X The expanding role of mitochondria in apoptosis. Genes Dev 2001; 15: 2922–2933.
Kroemer G, Reed JC Mitochondrial control of cell death. Nat Med 2000; 6: 513–519.
Li P, Nijhawan D, Budihardjo I, et al Cytochrome c and dATP dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997; 91: 479–489.
Zou H, Henzel WJ, Liu X, Lutschg A, Wang X Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c dependent activation of caspase-3. Cell 1997; 90: 405–413.
Du C, Fang M, Li Y, Li L, Wang X Smac, a mitochondrial protein, that promotes cytochrome c dependent caspase activation by eliminating IAP inhibition. Cell 2000; 102: 33–42.
Chai J, Du C, Wu JW, Kyin S, Wang X, Shi Y Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature 2000; 406: 855–862.
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999; 397:441–446.
Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, Ferri KF, Zamzami N, Wakeham A, Hakem R, Yoshida H, Kong YY, Mak TW, Zuniga-Pflucker JC, Kroemer G, Penninger JM Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 2001; 410: 549–554.
Wei MC, Zong WX, Cheng EH, et al Pro-apoptotic Bax and Bak: A requisite gateway to mitochondrial dysfunction and death. Science 2001; 292: 727–730.
Lindsten T, Ross AJ, King A, et al The combined functions of pro-apoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol Cell 2000; 6: 1389–1399.
Nagata S, Golstein P The Fas death factor. Science 1995; 267: 1449–1456.
Nagata S Fas ligand-induced apoptosis. Annu Rev Genet 1999; 33: 29–55.
Peter ME, Krammer PH Mechanisms of CD95 (APO-1/Fas)-mediated apoptosis. Curr Opin Immunol 1998; 10: 545–551.
Krammer PH CD95's deadly mission in the immune system. Nature 2000; 407: 789–795.
Hsu H, Xiong J, Goeddel DV The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell 1995; 81: 495–504.
Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM FADD, a novel death domain containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 1995; 81: 505–512.
Fulda S, Meyer E, Debatin KM Metabolic inhibitors sensitize for CD95 (APO-1/Fas)-induced apoptosis by down-regulating Fas-associated death domain-like interleukin 1-converting enzyme inhibitory protein expression. Cancer Res 2000; 60: 3947–3956.
Algeciras-Schimnich A, Shen L, Barnhart BC, Murmann AE, Burkhardt JK, Peter ME Molecular ordering of the initial signaling events of CD95. Mol Cell Biol 2002; 22: 207–220.
Scaffidi C, Fulda S, Srinivasan A, et al Two CD95 (APO-1/Fas) signaling pathways. Embo J 1998; 17: 1675–1687.
Li H, Zhu H, Xu CJ, Yuan J Cleavage of BID by caspase-8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998; 94: 491–501.
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 1998; 94: 481–490.
Santore MT, McClintock DS, Lee VY, Budinger GR, Chandel NS Anoxia-induced apoptosis occurs through a mitochondria dependent pathway in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 2002; 282: L727–734.
McClintock DS, Santore MT, Lee VY, Brunelle J, Budinger GR, Zong WX, Thompson CB, Hay N, Chandel NS Bcl-2 family members and functional electron transport chain regulate oxygen deprivation induced cell death. Mol Cell Biol 2002; 22: 94–104.
Parsadanian AS, Cheng Y, Keller-Peck CR, Holtzman DM, Snider WD Bcl-XL is an anti-apoptotic regulator for postnatal CNS neurons. J Neurosci 1998; 18: 1009–1019.
Shimizu S, Eguchi Y, Kosaka H, Kamiike W, Matsuda H, Tsujimoto Y Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-XL. Nature 1995; 374: 811–813.
Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 1996; 379: 88–91.
Saikumar P, Dong Z, Patel Y, Hall K, Hopfer U, Weinberg JM, Venkatachalam MA Role of hypoxia-induced Bax translocation and cytochrome c release in reoxygenation injury. Oncogene 1998; 17: 3401–3415.
Soengas MS, Alarcon RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW, Lowe SW Apaf-1 and caspase-9 in p53 dependent apoptosis and tumor inhibition. Science 1999; 284: 156–159.
Brunelle JK, Chandel NS. Unpublished data.
Kennedy SG, Kandel ES, Cross TK, Hay N Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol 1999; 19: 5800–5810.
Datta SR, Brunet A, Greenberg ME Cellular survival: A play in three Akts. Genes Dev 1999; 13: 2905–2927.
Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 1997; 275: 661–665.
Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N The PI-3 kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev 1997; 11: 701–713.
Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC Regulation of cell death protease caspase-9 by phosphorylation. Science 1998; 282: 1318–1321.
del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G Interleukin-3 induced phosphorylation of BAD through the protein kinase Akt. Science 1997; 278: 687–689.
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999; 96: 857–868.
Gottlob K, Kennedy S, Kandel E, Robey RB, Hay N Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes and Dev 2001; 15: 1406–1418.
Plas DR, Talapatra S, Edinger AL, Rathmell JC, Thompson CB Akt and Bcl-XL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J Biol Chem 2001; 276: 12041–12048.
Pastorino JG, Shulga N, Hoek JB Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem 2002; 277: 7610–7618.
di Cristofano A, Pandolfi PP The multiple roles of PTEN in tumor suppression. Cell 2000; 100: 387–390.
di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP PTEN is essential for embryonic development and tumour suppression. Nat Genet 1998; 19: 348–355.
Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW Negative regulation of PKB/Akt dependent cell survival by the tumor suppressor PTEN. Cell 1998; 95: 29–39.
Matsui T, Li L, del Monte F, Fukui Y, Franke TF, Hajjar RJ, Rosenzweig A Adenoviral gene transfer of activated phosphatidylinositol-3′kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation 1999; 100: 2373–2379.
Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, Gottschalk AR, Ryan HE, Johnson RS, Jefferson AB, Stokoe D, Giaccia AJ Loss ofPTENfacilitates HIF-1-mediated gene expression. Genes Dev 2000; 14: 391–396.
Mazure NM, Chen EY, Laderoute KR, Giaccia AJ Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol-3 kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood 1997; 90: 3322–3331.
Jiang BH, Zheng JZ, Aoki M, Vogt PK Phosphatidylinositol-3 kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci USA 2000; 97: 1749–1753.
Harris AL Hypoxia:Akey regulatory factor in tumour growth. Nature Rev Cancer 2002; 2: 38–47.
Alarcon RM, Denko NC, Giaccia AJ Genetic determinants that influence hypoxia-induced apoptosis. Novartis Found Symp 2001; 240: 115–128.
Schuler M, Green DR Mechanisms of p53 dependent apoptosis. Biochem Soc Trans 2001; 29: 684–688.
Graeber TG, Peterson JF, Tsai M, Monica K, Fornace AJ, Jr, Giaccia AJ Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low oxygen conditions is independent of p53 status. Mol Cell Biol 1994; 14: 6264–6277.
Koumenis C, Alarcon R, Hammond E, Sutphin P, Hoffman W, Murphy M, Derr J, Taya Y, Lowe SW, Kastan M, Giaccia A Regulation of p53 by hypoxia: Dissociation of transcriptional repression and apoptosis from p53 dependent transactivation. Mol Cell Biol 2001; 21: 1297–1310.
Sansome C, Zaika A, Marchenko ND, Moll UM Hypoxia death stimulus induces translocation of p53 protein to mitochondria. Detection by immunofluorescence on whole cells. FEBS Lett 2001; 488: 110–115.
Schmaltz C, Hardenbergh PH, Wells A, Fisher DE Regulation of proliferation-survival decisions during tumor cell hypoxia. Mol Cell Biol 1998; 18: 2845–2854.
Wang GL, Jiang B-H, Rue EA, Semenza GL Hypoxiainducible factor-1 is a basic helix-loop-helix PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 1995; 92: 5510–5514.
Semenza GL Regulation of mammalian O2 homeostasis by hypoxia-inducible factor-1. Annu Rev Cell Dev Biol 1999; 15: 551–578.
Bruick RK Expression of the gene encoding the pro-apoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci USA 2000; 97: 9082–9087.
Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL HIF-1 dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res 2001; 61: 6669–6673.
Ray R, Chen G, Vande Velde C, Cizeau J, Park JH, Reed JC, Gietz RD, Greenberg AH BNIP3 heterodimerizes with Bcl-2/Bcl-XL and induces cell death independent of a Bcl-2 homology 3 (BH3) domain at both mitochondrial and nonmitochondrial sites. J Biol Chem 2000; 275: 1439–1448.
Chen G, Cizeau J, Vande Velde C, Park JH, Bozek G, Bolton J, Shi L, Dubik D, Greenberg A NIX and Nip3 form a subfamily of pro-apoptotic mitochondrial proteins. J Biol Chem 1999; 274: 7–10.
Vande Velde C, Cizeau J, Dubik D, Alimonti J, Brown T, Israels S, Hakem R, Greenberg AH BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol Cell Biol 2000; 20: 5454–5468.
Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E, Keshet E Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998; 394: 485–490.
Akakura N, Kobayashi M, Horiuchi I, Suzuki A, Wang J, Chen J, Niizeki H, Kawamura KI, Hosokawa M, Asaka M Constitutive expression of hypoxia inducible factor-1α renders pancreatic cancer cells resistant to apoptosis induced by hypoxia and nutrient deprivation. Cancer Res 2001; 61: 6548–6554.
Maltepe E, Chandel NS. Unpublished data.
Ozawa K, Kuwabara K, Tamatani M, Takatsuji K, Tsukamoto Y, Kaneda S, Yanagi H, Stern DM, Eguchi Y, Tsujimoto Y, Ogawa S, Tohyama M 150-kDa oxygen-regulated protein (ORP150) suppresses hypoxia-induced apoptotic cell death. J Biol Chem 1999; 274: 6397–6404.
Dong Z, Venkatachalam MA, Wang J, Patel Y, Saikumar P, Semenza GL, Force T, Nishiyama J Up-regulation of apoptosis inhibitory protein IAP-2 by hypoxia. HIF-1-independent mechanisms. J Biol Chem 2001; 276: 18702–18709.
Shoshani T, Faerman A, Mett I, Zelin E, Tenne T, Gorodin S, Moshel Y, Elbaz S, Budanov A, Chajut A, Kalinski H, Kamer I, Rozen A, Mor O, Keshet E, Leshkowitz D, Einat P, Skaliter R, Feinstein E Identification of a novel hypoxia inducible factor-1 responsive gene, RTP801, involved in apoptosis. Mol Cell Biol 2002; 22: 2283–2293.
Tamatani M, Matsuyama T, Yamaguchi A, Mitsuda N, Tsukamoto Y, Taniguchi M, Che YH, Ozawa K, Hori O, Nishimura H, Yamashita A, Okabe M, Yanagi H, Stern DM, Ogawa S, Tohyama M ORP150 protects against hypoxia/ischemia induced neuronal death. Nat Med 2001; 7: 317–323.
Jacobson MD, Burne JF, King MP, Miyashita T, Reed JC, Raff MC Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature 1993; 361: 365–369.
Wang J, Silva JP, Gustafsson CM, Rustin P, Larsson NG Increased in vivo apoptosis in cells lacking mitochondrial DNA gene expression. Proc Natl Acad Sci USA 2001; 98: 4038–4043.
Marchetti P, Susin SA, Decaudin D, Gamen S, Castedo M, Hirsch T, Zamzami N, Naval J, Senik A, Kroemer G Apoptosis-associated derangement of mitochondrial function in cells lacking mitochondrial DNA. Cancer Res 1996; 56: 2033–2038.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brunelle, J.K., Chandel, N.S. Oxygen deprivation induced cell death: An update. Apoptosis 7, 475–482 (2002). https://doi.org/10.1023/A:1020668923852
Issue Date:
DOI: https://doi.org/10.1023/A:1020668923852