Abstract
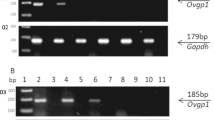
Purpose : To examine the in vivo expression of oviductin mRNA at different stages of the human female reproductive cycle including pregnancy and after menopause.
Methods : Oviducts were obtained from 25 women in normal menstrual cycle, 5 in early pregnancy, 5 undergoing postpartum sterilization, and 4 menopausal women. The oviductal mucosal tissue was isolated and oviductin mRNA was assessed using reverse-transriptase-polymerase chain reaction (RT-PCR); its correlation with various hormones was assessed.
Results : Oviductin mRNA was detected throughout the menstrual cycle, highest in the periovulatory period. It continued to be expressed in early pregnancy but was absent in the postpartum period and after menopause.
Conclusions : The production and function of oviductin at different stages of human reproductive cycle including pregnancy is not well known. Its highest expression at the time of ovulation is consistent with a supportive role in fertilization and early embryo development.
Similar content being viewed by others
REFERENCES
Boatman DE: Oviductal modulators of sperm fertilizing ability. In Fertilization in Mammals, BD Bavister, JM Cummins, E Roldan (eds), Norwell, MA, Serono Symposia USA, 1990, pp 223–238
Boatman DE: Responses of gametes to the oviductal environment. Hum Reprod 1997;12:133–149
Boatman DE, Magnoni GE: The role of an estrogen dependent glycoprotein, oviductin, in fertilization in the golden hamster. In Proceedings of the Twenty Sixth Annual Conference, Australia Society of Reproductive Biology, Brisbane, Australia, 1994, p 105
Anderson SH, Killian GJ: Effect of macromolecules from oviductal conditioned medium on bovine sperm motion and capacitation. Biol Reprod 1994;51:795–799
King RS, Killian GJ: Purification of bovine estrus-associated protein and localization of binding on sperm. Biol Reprod 1994;51:4–42
Boice ML, McCarthy TJ, Mavrogianis PA, Fazleabas AT, Verhage HG:Localisation of oviductal glycoproteins within the zona pellucida and perivitelline space of ovulated ova and early embryos in baboons (Papio anubis). Biol Reprod 1990;43:340–346
O'Day-Bowman MB, Mavrogianis PA, Reuter LM, Johnson DE, Fazleabas AT, Verhage HG: Association of oviduct specific glycoproteins with human and baboon (Papio anubis) ovarian oocytes and enhancement of human sperm binding to human hemizonae following in vitro incubation. Biol Reprod 1996;54:60–69
Hill JL, Walker SK, Brown GH, Nancarrow CD: The effects of an ovine oviductal estrus-associated glycoprotein on early embryo development. Theriogenology 1996;46:1367–1377
Bongso TA, Fong CY, Ng SC, Ratnam SS: Blastocyst transfer in human in vitro fertilization: The use of embryo co-culture. Cell Biol Int 1994;18:1181–1189
Kaufman RA, Menezo Y, Hazout A: Cocultured blastocyst cryopreservation: Experience of more than 500 transfer cycles. Fertil Steril 1995;64:1125–1129
Hill JL, Walker SK, Brown GH, Nancarrow CD: The effects of an estrus-associated oviductal glycoprotein on the in vitro fertilization and development of ovine oocytes matured in vitro. Theriogenology 1996;46:1379–1388
Vansteenbrugge A, Van Langendonckt A, Massip A, Dessy F: Effect of estrus-associated glycoprotein and tissue inhibitor of metalloproteinase-1 secreted by oviduct cells on in vitro bovine embryo development. Mol Reprod Dev 1997;46:527–534
Malette B, Paquette Y, Merlen Y, Bleau G: Oviductins possess chitinase-and mucin-like domains: A lead in the search for the biological function of these oviduct-specific ZP-associatins glycoproteins. Mol Reprod Dev 1995;41:384–397
Leveille MC, Roberts KD, Chevalier S, Shapdelaine V, Bleau G: Uptake of an oviductal antigen by the hamster zona pellucida. Biol Reprod 1987;36:227–238
Oliphant G, Ross PR: Demonstration of production and isoloation of three sulfated glycoproteins from the rabbit oviduct. Biol Reprod 1982;26:537–544
Oliphant G, Reynolds AB, Smith PF, Ross PR, Marta JS: Immunocytochemical localization and determination of hormone-induced synthesis of the sulfated oviductal glycoproteins. Biol Reprod 1984;31:165–174
Sutton R, Nancarrow CD, Wallace ALC, Rigby NW: Identifi-cation of an oestrus-associated glycoprotein in oviducal fluid of the sheep. J Reprod Fertil 1984;72:415–422
Buhi WC, Alvarez IM, Suldhipong V, Donnes-Smith MM: Identification and characterization of de-novo synthesized of porcine oviductal secretory proteins. Biol Reprod 1990;43:929–938
Boice ML, Geisert RD, Blair RM, Verhage HG: Identification and characterization of bovine oviductal glycoproteins synthesized at estrus. Biol Reprod 1990;43:457–565
Malayar JR, Hansen PJ, Buhi WC: Secretion of proteins by cultured bovine oviducts collected from estrus through early diestus. J Exp Zool 1988;238:345–353
Verhage HG, Fazleabas AT: The in-vitro synthesis of estrogendependent proteins by the baboon (Papio anubis) oviduct. Endocrinology 1988;123:552–558
Verhage HG, Fazleabas AT, Donnelly K: The in vitro synthesis and release of proteins by the human oviduct. Endocrinology 1988;122:1639–1645
Roux E, Kan FWK: Stage-specific immunolabelling for oviductin in the secretory granules of the oviductal epithelium of the golden hamster during the estrous cycle. Anat Rec 1995;241:369–376
Komiya H, Onuma T, Hiroi M, Araki Y: In situ localization of messenger ribonucleic acid for an oviduct-specific glycoprotein during various hormonal conditions in the golden hamster. Biol Reprod 1996;55:1107–1118
Roux E, Bleau G, Kan FWK: Fate of hamster oviductin in the oviduct and uterus during early gestation. Mol Reprod Dev 1997;46:306–376
Buhi WC: Cyclic changes in the oviduct during fertilization and early cleavage-stage embryonic development. Arch Anim Breeding 1996;39:15–25
Geisert RD, Buhi SC, Fields M: Synthesis and release of polypeptides by bovine oviductal tissue in culture. Biol Reprod 1987;36(Suppl):84
Arias EB, Verhage HG, Jaffe RC: Complementary deoxyribonucleic acid cloning and molecular characterization of an estrogen-dependent human oviductal glycoprotein. Biol Reprod 1994;51:685–694
Sun T, Lei ZM, Rao CV: A novel regulation of the oviductal glycoprotein gene expression by luteinizing hormone in bovine tubal epithelial cells. Molec Cell Endocrin 1997;131:97–108
Briton-Jones C, Lok IH, Yuen PM, Chiu TTY, Cheung LP, Haines C: Regulation of human oviductin mRNA expression in-vivo. Fertil Steril 2001;75:942–946
Soutar RL, Dillon J, Ralston SH: Control genes for reversetranscription-polymerase chain reaction: A comparison of beta-actin and glyceraldehyde phosphate dehydrogenase. Br J Haematol 1997;97:247–248
Jaffe RC, Arias EB, O'Day-Bowman MB, Donnelly KM, Mavrogianis PA, Verhage HG: Regional distribution and hormonal control of estrogen-dependent oviduct-specific glycoprotein messenger ribonucleic acid in the baboon (Papio anubis). Biol Reprod 1996;55:421–426
Sambrook J, Fritsch EF, Maniatis: Molecular Cloning: A Laboratory Manual, 2nd edn. New York, Cold Spring Harbour Laboratory Press, 1989
Verhage HG, Mavrogianis PA, Boomsma RA, Schmidt A, Brenner RM, Slayden OV: Immunologic and molecular characterization of an estrogen-dependent glycoprotein in the Rhesus (Macaca mulatta) oviduct. Biol Reprod 1997;57:525–531
Lapensee L, Paquette Y, Bleau G: Allelic polymorphism and chromosomal localization of the human oviductin gene (MUC9). Fertil Steril 1997;68:702–708
Paquette Y, Merlen Y, Malette B, Bleau G: Allelic polymorphism in the hamster oviductin gene is due to a variable number of mucin-like tandem repeats. Mol Reprod Dev 1995;42:388–396
Lagow E, DeSouza MM, Carson DD: Mammalian reproductive tract mucins. Hum Reprod Update 1999;5:280–292
Malette B, Bleau G: Biochemical characterization of hamster oviductin as a sulphated zona pellucida-binding glycoprotein. Biochem J 1993;295:437–445
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lok, I.H., Briton-Jones, C.M., Yuen, P.M. et al. Variable Expression of Oviductin mRNA at Different Stages of Human Reproductive Cycle. J Assist Reprod Genet 19, 569–576 (2002). https://doi.org/10.1023/A:1021263132176
Issue Date:
DOI: https://doi.org/10.1023/A:1021263132176