Abstract
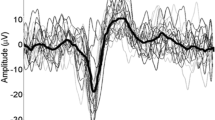
The study aim was to evaluate the effect of different attentional tasks on the amplitudes and latencies of painful and non-painful contact heat evoked potentials (CHEPs). CHEPs were recorded in 12 healthy subjects during two experimental conditions, in which attention was oriented towards the intensity and the distress caused by the stimuli and were compared with CHEPs recorded during a neutral condition. The painful heat stimulation produced a negative potential at Cz vertex with a latency around 540 ms (Cz/N540), a positive peak at Cz electrode around 730 ms (Cz/P730) and, lastly, a positive peak around 1000 ms (Pz/P1000) in the Pz traces. The Cz/P730 wave was significantly higher in amplitude only during the painful stimulation and is probably related to coding the nociceptive activity. Varying the attentional target towards different properties of the stimulus did not cause any significant change in CHEP responses amplitude and latencies compared with the neutral condition. Our results suggest that CHEPs represent a reliable functional measure of the nociceptive pathways and that they are generated by the activation of different cerebral areas involved in pain processing. The high activation level of each of these area or their spatial neighbouring might explain the strong similarity of CHEP components recorded during different attentional manipulations.
Similar content being viewed by others
References
Ahles, T.A., Blanchard, E.B. and Leventhal, H. Cognitive control of pain: attention to the sensory aspects of the cold pressor stimulus. Cogn. Ther. Res., 1983, 7: 159–177.
Arendt-Nielsen, L., Zachariae, R. and Bjerring, P. Quantitative evaluation of hypnotically suggested hyperaesthesia and analgesia by painful laser stimulation. Pain, 1990, 42: 243–251.
Arendt-Nielsen, L., Nielsen, J., Svensson, P., Shimojo, M. and Chen, C.A.N. Evoked potentials to painful heat stimulation. In: C. Barber, G.G. Celesia, I. Hashimoto and R. Kakigi (Eds.), Functional Neuroscience: Evoked Potentials and Magnetic Fields (EEG suppl. 49), Amsterdam, Elsevier Science, 1999: 261–266.
Barber, J. and Mayer, D. Evaluation of the efficacy and neural mechanism of a hypnotic analgesia procedure in experimental and clinical dental pain. Pain, 1977, 4: 41–48.
Beydoun, A., Morrow, T.J., Shen, J.F. and Casey, K.L. Variability of laser-evoked potentials: attention, arousal and lateralized differences. Electroencephalogr. Clin. Neurophysiol., 1993, 88: 173–181.
Bragard, D., Chen, C.A.N. and Plaghki, L. Direct isolation of ultra-late (C-fibre) evoked brain potentials by CO2 laser stimulation of tiny cutaneous surface areas in man. Neurosci. Lett., 1996, 209: 81–84.
Bromm, B. and Treede, R.D. Pain related cerebral potentials: late and ultralate components. Int. J. Neurosci., 1987, 33: 15–23.
Bromm, B. and Lorenz, J. Neurophysiological evaluation of pain. Electroencephalogr. Clin. Neurophysiol., 1998, 107: 227–253.
Bushnell, M.C., Duncan, G.H., Dubner, R., Jones, R.L. and Maixner, W. Attentional influences on noxious and innocuous cutaneous heat detection in humans and monkeys. J. Neurosci., 1985, 5: 1103–1110.
Casey, K.L., Morrow, T.J., Lorenz, J. and Minoshima, S. Temporal and spatial dynamics of human forebrain activity during heat pain: analysis by positron emission tomography. J. Neurophysiol., 2001, 2: 951–959.
Chen, A.C.N., Niddam, D.M. and Arendt-Nielsen, L. Contact heat evoked potentials as a valid means to study nociceptive pathways in human subjects. Neurosci. Lett., 2001, 316: 79–82.
Davis, K.D., Hutchison, W.D., Lozano, A.M. and Dostrovsky, J.O. Altered pain and temperature perception following cingulotomy and capsulotomy in a patient with schizoaffective disorder. Pain, 1994, 59: 189–199.
De Pascalis, V., Magurano, M.R., Bellusci, A. and Chen, A.C.N. Somatosensory event-related potential and autonomic activity to varying pain reduction cognitive strategies in hypnosis. Clin. Neurophysiol., 2001, 112: 1475–1485.
García-Larrea, L., Peyron, R., Laurent, B. and Mauguiè re, F. Association and dissociation between laser-evoked potentials and pain perception. Neuroreport, 1997, 8: 3785–3789.
Harkins, S.W., Price, D.D. and Katz, M.A. Are cerebral evoked potentials reliable indices of first or second pain? In: J.J. Bonica, U. Lindblom and A. Iggo (Eds.), Advances in Pain research and Therapy. New York, Raven Press, 1983, 5: 185–191.
Harkins, S.W., Price, D.D., Roy, A., Itskovich, V.V. and Ding-Yu, F. Somatosensory evoked potentials associated with thermal activation of type II Ad mechanoheat nociceptive afferents. Intern. J. Neurosci., 2000, 104: 93–111.
Hilgard, E.R. and Hilgard, J.R. Hypnosis in the relief of pain. Br. J. Psychiat., 1985, 146: 325–335.
Hofbauer, R.K. Rianville, P., Duncan, G.H. and Bushnell, M.C. Cortical representation of the sensory dimension of pain. J. Neurophysiol., 2001, 86: 402–411.
Holmes, J.D., Hekmat, H. and Mozingo, B.S. Cognitive and behavioral regulation of pain: the facilitative effects of analgesic suggestions. Psychol. Rec., 1983, 33: 151–159.
Hutchison, W.D., Davis, K.D., Lozano, A.M., Tasker, R.R. and Dostrovsky, J.O. Pain-related neurons in the human cingulate cortex. Nat. Neurosci., 1999, 2: 403–405.
Itskovich, V.V., Ding-Yu, F. and Harkins, S.W. Psychophysiological and psychophysical responses to experimental pain induced by two types of cutaneous thermal stimuli. Intern. J. Neurosci., 2000, 105: 63–75.
Kanda, M., Shindo, K., Xu, X., Fujiwara, N., Ikeda, A., Nagamine, T. and Shibasaki, H. Cortical mechanisms underlying point localization of pain spot as studied by event-related potentials following CO2 laser stimulation in man. Exp. Brain. Res., 1999, 127(2): 131–140.
Kwan, C.H., Crawley, A.P., Mikulis, D.J. and Davis, K.D. An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain, 2000, 85: 359–374.
Lehmann, D. and Skrandies, W. Spatial analysis of evoked potentials in man-a review. Prog. Neurobiol., 1984, 23: 227–250.
Levine, J.D., Gordon, N.C., Smith, R. and Fields, H.L. Post-operative pain: effect of extent of injury and attention. Brain Res., 1982, 234: 500–504.
Magerl, W., Ali, Z., Ellrich, J., Meyer, R.A. and Treede, R.D. Cand Ad-fiber components of heat-evoked cerebral potentials in healthy human subjects. Pain, 1999, 82: 127–137.
McCaul, K.D. and Haugtvedt, C. Attention, distraction and cold-pressor pain. J. Pers. Soc. Psychol., 1982, 43: 154–162.
Melzack, R. and Wall, P.D. Pain mechanisms: a new theory. Science, 1965, 150: 971–979.
Merskey, H. The definition of pain. Eur. J. Psychiatry, 1991, 6: 153–159.
Miltner, W., Johnson, R., Braun, C. and Larbig, W. Somatosensory event-related potentials to painful and non-painful stimuli: effects of attention. Pain, 1989, 38: 303–312.
Miron, D., Duncan, G.H. and Bushnell, M.C. Effects of attention on the intensity and unpleasantness of thermal pain. Pain, 1989, 39: 345–352.
Nielsen, J. and Arendt-Nielsen, L. Spatial summation of heat induced painwithin and between dermatomes. Somatosens. Motor Res., 1997, 14: 119–125.
Opsommer, E., Weiss, T., Plaghki, L. and Miltner, W. Dipole analysis of ultralate (C-fibres) evoked potentials after laser stimulation of tiny cutaneous surface areas in humans. Neurosci. Lett., 2001, 298: 41–44.
Plaghki, L., Delisle, D. and Godfraind, J.M. Heterotopic nociceptive conditioning stimuli and mental task modulate differently the perception and physiological correlates of short CO2 laser stimuli. Pain, 1994, 57: 181–192.
Peyron, R., García-Larrea, L., Grégoire, M.C., Costes, N., Convers, P., Lavenne, F., Mauguiè re, F., Michel, D. and Laurent, B. Haemodynamic brain responses to acute pain in humans. Sensory and attentional networks. Brain, 1999, 122: 1765–1779.
Price, D.D., Harkins, S.W., Rafii, A. and Price, C. A simultaneous comparison of fentanyl's analgesic effects on experimental and clinical pain. Pain, 1986, 24: 197–203.
Price, D.D. Psychological and neural mechanisms of the affective dimension of pain. Science, 2000, 288: 1769–1772.
Rainville, P., Duncan, G.H., Price, D.D., Carrier, B. and Bushnell, M.C. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science, 1997, 277: 968–971.
Rainville, P., Carrier, B., Hofbauer, R.K., Bushnell, M.C. and Duncan, G.H. Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain, 1999, 82: 159–171.
Sawamoto, N., Honda, M., Okada, T., Hanakawa, T., Kanda, M., Fukuyama, H., Konishi, J. and Shibasaki, H. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal Operculum/Posterior insula: an event related functional magnetic resonance imaging study. J. Neurosci., 2000, 20: 7438–7445.
Siedenberg, R. and Treede, R.D. Laser-evoked potentials: exogenous and endogenous components. Electroencephalogr. Clin. Neurophysiol., 1996, 100: 240–249.
Sikes, R.W. and Vogt, B.A. Nociceptive neurons in area 24 of rabbit cingulated cortex. J. Neurophysiol., 1992, 68: 1720–1732.
Talbot, J.D., Villemure, J.G., Bushnell, M.C. and Duncan, G.H. Evaluation of pain perception after capsulotomy: a case report. Somatosens. Mot. Res., 1995, 12: 115–126.
Tolle, T.R., Kaufmann, T., Siessmeier, T., Lautenbacher, S., Berthele, A., Munz, F., Zieglgansberger, W., Willoch, F., Schwaiger, M., Conrad, B. and Bartenstein, P. Region-specific encoding of sensory and affective components of pain in the human brain: a positron tomography correlation analysis. Ann. Neurol., 1999, 45: 40–47.
Tran, T.D., Lam, K., Hoshiyama, M. and Kakigi, R. A new method for measuring the conduction velocities of Abeta-, Adelta-and C-fibers following electric and C02 laser stimulation in humans. Neurosci. Lett., 2001, 301: 187–190.
Treede, R.D., Meyer, R.A. and Lesser, R.P. Similarity of threshold temperatures for first pain sensation, laser-evoked potentials and nociceptor activation. In: G.F. Gebhart, D.L. Hammond and T.S. Jensen (Eds.), Proceedings of the 7th World Congress on Pain. Progress in pain research and Management, Seattle, WA, IASP Publication, 1994, 2: 857–865.
Treede, R.D., Kenshalo, D.R., Gracely, R.H. and Jones, A.K.P. The cortical representation of pain. Pain, 1999, 79: 105–111.
Valeriani, M., Le Pera, D., Niddam, D., Chen, A.C.N. and Arendt-Nielsen, L. Dipolar modelling of the scalp evoked potentials to painful contact heat pain stimulation of the human skin. Neurosci Lett., 2002a, 31: 44–48.
Valeriani, M., Restuccia, D., Le Pera, D., De Armas, L., Maiese, T. and Tonali, P. Attention-related modifications of ultra-late CO2 laser evoked potentials to human trigeminal nerve stimulation. Neurosci. Lett., 2002b, 329: 329–332.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Le Pera, D., Valeriani, M., Niddam, D. et al. Contact Heat Evoked Potentials to Painful and Non-Painful Stimuli: Effect of Attention Towards Stimulus Properties. Brain Topogr 15, 115–123 (2002). https://doi.org/10.1023/A:1021472524739
Issue Date:
DOI: https://doi.org/10.1023/A:1021472524739