Abstract
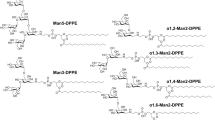
Purpose. To improve target specificity and uptake of liposomes by macrophages, one can improve high-affinity receptor binding to mannose determinants with their 175-kDa mannose receptor (MR), which is mainly influenced by the length and flexibility of the spacer between the carbohydrate head group and liposome surface. Liposomes containing alkylmannosides with hydrophilic spacers 0 to 8 ethyleneoxy units (EO) long (Man0...Man8) were used to investigate systematically the effects of spacer length on liposome-cell interactions.
Methods. Concanavalin A (ConA)-induced liposome aggregation was studied by turbidity measurement and cell uptake using PMA-induced HL-60 cells or native human macrophages by determining 6-CF after cell lysis or NBD-fluorescence with flow cytometry. Detection of MR in native cell populations was carried out by an antibody assay using flow cytometry; MR-representing cells were selected analytically.
Results. Liposomes containing mannosides with more than one EO spacer length were specifically aggregated by ConA, indicating accessibility of the carbohydrate ligands of these derivatives. Increase in EO spacer units of incorporated mannosides (two or more EO) led to suppression of cellular uptake of mannosylated liposomes by phago- cytes lacking MR (HL60, U937). The extent of suppression increased with spacer length. Liposome uptake by native macrophages expressing MR was, on the contrary, improved, particularly by Man6 and Man8.
Conclusions. Uptake of liposomes modified with Man6 or Man8 by native cells was enhanced but did not reach an optimum. Thus, Man6, Man8, and mannosides with even longer spacer arms are of potential use in receptor-mediated targeting.
Similar content being viewed by others
REFERENCES
S. E. Pontow, V. Kery, and P. D. Stahl. Mannose receptor. Int. Rev. Cytol. 137B:221-244 (1992).
C. R. Alving. Theoretical basis for development of liposomes as carriers for vaccines. In D. D. Lasic and D. Papahadjopoulos (eds.), Medical Applications of Liposomes. Elsevier Science, Amsterdam, 1998, pp. 145-163.
C. Popescu, V. Alexandrescu, D. Ivanov, G. Manda, and M. Neagu. Mannose-coated liposomes as adjuvant for influenza virus vaccine: stimulation of mouse peritoneal macrophages respiratory burst. J. Med. Biochem. 4:51-55 (2000).
J. Sunamoto and K. Iwamoto. Protein anchored and polysaccharide-anchored liposomes as drug carriers. Crit. Rev. Ther. Drug Carrier Syst. 2:117-136 (1986).
M. N. Jones. Carbohydrate mediated liposomal targeting and drug delivery. Adv. Drug Deliv. Rev. 13:215-250 (1994).
G. Molema and D. K. F. Meijer. Targeting of drugs to various blood cell types using (neo-) glycoproteins, antibodies and other protein carriers. Adv. Drug Deliv. Rev. 14:25-50 (1994).
G. Barratt, J.-P. Tenu, A. Yapo, and J.-F. Petit. Preparation and characterisation of liposomes containing mannosylated phospholipids capable of targetting drugs to macrophages. Biochim. Biophys. Acta 862:153-164 (1986).
P. Opanasopit, Y. Higuchi, S. Kawakami, F. Yamashita, M. Nishikawa, and M. Hashida. Involvement of serum mannan binding proteins and mannose receptors in uptake of mannosylated liposomes by macrophages. Biochim. Biophys. Acta 1511:135-145 (2001).
S. Kawakami, J. Wong, A. Sato, Y. Hattori, F. Yamashita, and M. Hashida. Biodistribution characteristics of mannosylated, fucosylated and galactosylated liposomes in mice. Biochim. Biophys. Acta 1524:258-265 (2000).
N. Garcon, G. Gregoriadis, and M. Taylor. and J. Summerfield. Mannose-mediated targeted immunoadjuvant action of liposomes. Immunology 64:743-745 (1988).
M. Dutta, R. Bandyopadhyay, and M. K. Basu. Neoglycosylated liposomes as efficient ligands for the evaluation of specific sugar receptors on macrophages in health and in experimental leishmaniasis. Parasitology 109:139-147 (1994).
P. Ghosh and B. K. Bachhawat. Grafting of different glycosides on the surface of liposomes and its effect on the tissue distribution of 125I-labelled gamma-globulin encapsulated in liposomes. Biochim. Biophys. Acta 632:562-572 (1980).
I. Ahmad, A. K. Sarkar, and B. K. Bachhawat. Mannosylated liposome-mediated delivery of amphotericin-B in the control of experimental aspergillosis in BALB/c mice. J. Clin. Biochem. Nutr. 10:171-179 (1991).
G. Banerjee, G. Nandi, S. B. Mahato, A. Pakrashi, and M. K. Basu. Drug delivery system: targeting of pentamidines to specific sites using sugar grafted liposomes. J. Antimicrob. Chemother. 38:145-150 (1996).
P. Opanasopit, M. Sakai, M. Nishikawa, S. Kawakami, F. Yamashita, and M. Hashida. Inhibition of liver metastasis by targeting of immunomodulators using mannosylated liposome carriers. J. Controlled Release 80:283-294 (2002).
A. Duffels, L. G. Green, S. V. Ley, and A. D. Miller. Synthesis of high-mannose type neoglycolipids: active targeting of liposomes to macrophages in gene therapy. Chemistry 6:1416-1430 (2000).
S. Kawakami, A. Sato, M. Nishikawa, F. Yamashita, and M. Hashida. Mannose receptor-mediated gene transfer into macrophages using novel mannosylated cationic liposomes. Gene Ther. 7:292-299 (2000).
A. Sato, S. Kawakami, M. Yamada, F. Yamashita, and M. Hashida. Enhanced gene transfection in macrophages using mannosylated cationic liposome-polyethylenimine-plasmid DNA complexes. J. Drug Target. 9:201-207 (2001).
C. D. Muller and F. Schuber. Neo-mannosylated liposomes: synthesis and interaction with mouse Kupffer cells and resident peritoneal macrophages. Biochim. Biophys. Acta 986:97-105 (1989).
M. Domurado, D. Domurado, S. Vansteenkiste, A. De Marre, and E. Schacht. Glucose oxidase as a tool to study in vivo the interaction of glycosylated polymers with the mannose receptor of macrophages. J. Controll. Rel. 33:115-123 (1995).
Y. Ohsumi, C. A. Hoppe, T. Ogawa, and Y. C. Lee. Enhancement of macromolecular ligand binding by rabbit alveolar macrophages by mannose oligosaccharides and related compounds. Arch. Biochem. Biophys. 260:241-249 (1988).
W. W. Liang, X. Shi, D. Deshpande, C. J. Malanga, and Y. Rojanasakul. Oligonucleotide targeting to alveolar macrophages by mannose receptor-mediated endocytosis. Biochim. Biophys. Acta 1279:227-234 (1996).
M. Monsigny, A.-C. Roche, P. Midoux, and R. Mayer. Glycoconjugates as carriers for specific delivery of therapeutic drugs and genes. Adv. Drug Deliv. Rev. 14:1-24 (1994).
E. A. L. Biessen, F. Noorman, M. E. van Teijlingen, J. Kuiper, M. Barrett-Bergshoeff, M. K. Bijsterbosch, D. C. Rijken, and T. J. C. van Berkel. Lysine-based cluster mannosides that inhibit ligand binding to the human mannose receptor at nanomolar concentration. J. Biol. Chem. 271:28024-28030 (1996).
S. K. Chatterjee and P. Nuhn. Stereoselective α-glycosidation using FeCl3 as Lewis acid catalyst. J. Chem. Soc. Chem. Commun. 1729-1730 (1998).
F. Wilhelm, S. K. Chatterjee, B. Rattay, P. Nuhn, R. Benecke, and J. Ortwein. Synthesis of glycolipids as membrane-bound stabilizing carbohydrates. Liebigs Ann. 1673-1679 (1995).
G. Rovera, G. Santoli, and C. Damsky. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc. Natl. Acad. Sci. USA 76:2779-2783 (1979).
F. Noorman, E. A. M. Braat, M. Barrett-Bergshoeff, E. Barbe, A. van Leeuwen, J. Lindeman, and D. C. Rijken. Monoclonal antibodies against the human mannose receptor as a specific marker in flow cytometry and immunohistochemistry for macrophages. J. Leukoc. Biol. 61:63-72 (1997).
R. Sundler. Studies on the effective size of phospholipid headgroups in bilayer vesicles using lectin-glycolipid interaction as a steric probe. Biochim. Biophys. Acta 771:59-67 (1984).
H. Yoshioka, T. Ohmura, M. Hasegawa, S. Hirota, M. Makino, and M. Kamiya. Synthesis of galactose derivatives that render lectin-induced agglutinating ability to liposomes. J. Pharm. Sci. 82:273-275 (1993).
A. Sasaki, N. Murahashi, H. Yamada, and A. Morikawa. Syntheses of novel galactosyl ligands for liposomes and the influence of the spacer on accumulation in the rat liver. Biol. Pharm. Bull. 18:740-746 (1995).
T. M. Allen. The use of glycolipids and hydrophilic polymers in avoiding rapid uptake of liposomes by the mononuclear phagocyte system. Adv. Drug Deliv. Rev. 13:285-309 (1994).
H.-J. Gabius. Non-carbohydrate binding partners/domains of animal lectins. Int. J. Biochem. 26:469-477 (1994).
H. Debray, D. Decout, G. Strecker, G. Spik, and J. Montreuil. Specificity of twelve lectins towards oligosaccharides and glycopeptides related to N-glycosylproteins. Eur. J. Biochem. 117:41-55 (1981).
Y. Shinohara, H. Sota, F. Kim, M. Shimizu, M. Gotoh, M. Tosu, and Y. Hasegawa. Use of a biosensor based on surface plasmon resonance and biotinyl glycans for analysis of sugar binding specificities of lectins. J. Biochem. 117:1076-1082 (1995).
R. W. Jansen, G. Molema, T. L. Ching, R. Oosting, G. Harms, F. Moolenaar, M. J. Hardonk, and D. K. F. Meijer. Hepatic endocytosis of various types of mannose-terminated albumins. What is important, sugar recognition, net charge, or the combination of these features. J. Biol. Chem. 266:3343-3348 (1991).
V. Kery, J. J. Krepinsky, C. D. Warren, P. Capek, and P. D. Stahl. Ligand recognition by purified human mannose receptor. Arch. Biochem. Biophys. 298:49-55 (1992).
M. Monsigny, A.-C. Roche, and P. Midoux. Uptake of neoglycoproteins via membrane lectin(s) of L1210 cells evidenced by quantitative flow cytofluorometry and drug targeting. Biol. Cell 51:187-196 (1984).
H.-J. Gabius, U. Brinck, T. Lüsebrink, T. Ciesiolka, and S. Gabius. Glycopeptide-albumin derivative: it preparation and histochemical ligand properties. Histochem. J. 23:303-311 (1991).
K. Drickamer. Clearing up glycoprotein hormones. Cell 67:1029-1032 (1991).
K. Shimada, J. A. A. M. Kamps, J. Regts, K. Ikeda, T. Shiozawa, S. Hirota, and G. L. Scherphof. Biodistribution of liposomes containing synthetic galactose-terminated diacylglyceryl-poly(ethyleneglycol)s. Biochim. Biophys. Acta 1326:329-341 (1997).
A. Chakrabarti and S. K. Podder. Complex carbohydrate-lectin interaction at the interface: a model for cellular adhesion. I. Effect of vesicle size on the kinetics of aggregation between a fatty acid conjugate of lectin and a liposomal asialoganglioside. Biochim. Biophys. Acta 1024:103-110 (1990).
R. D. Astumian and Z. A. Schelly. Geometric effects of reduction and dimensionality in interfacial reactions. J. Am. Chem. Soc. 106:304-308 (1984).
G. A. Orr, R. R. Rando, and F. W. Bangerter. Synthetic glycolipids and the lectin-mediated aggregation of liposomes. J. Biol. Chem. 254:4721-4725 (1979).
C. E. Napper, M. H. Dyson, and M. E. Taylor. An extended conformation of the macrophage mannose receptor. J. Biol. Chem. 276:14759-14766 (2001).
F. Noorman, M. M. Barrett-Bergshoeff, E. A. L. Biessen, E. Van de Bilt, T. J. C. van Berkel, and D. C. Rijken. Cluster mannosides can inhibit mannose receptor-mediated tissue-type plasminogen activator degradation by both rat and human cells. Hepatology 26:1303-1310 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Engel, A., Chatterjee, S.K., Al-arifi, A. et al. Influence of Spacer Length on Interaction of Mannosylated Liposomes with Human Phagocytic Cells. Pharm Res 20, 51–57 (2003). https://doi.org/10.1023/A:1022294624256
Issue Date:
DOI: https://doi.org/10.1023/A:1022294624256