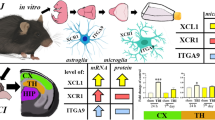
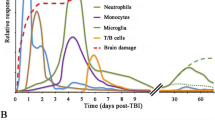
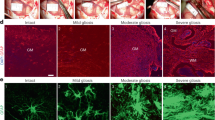
Abstract
A traumatic injury to the adult mammalian central nervous system (CNS), such as a stab wound lesion, results in reactive astrogliosis and the migration of hematogenous cells into the damaged neural tissue. The roles of cytokines and growth factors released locally by the damaged endogenous cells are recognized in controlling the cellular changes that occur following CNS injury. However, the role of chemokines, a novel class of chemoattractant cytokines, is only recently being studied in regulating inflammatory cell invasion in the injured/diseased CNS (1). The mRNAs for several chemokines have been shown to be upregulated in experimental allergic encephalomyelitis (EAE), an inflammatory demyelinating disease of the CNS, but chemokine expression in traumatic brain injury has not been studied in detail. Astrocytes have been demonstrated to participate in numerous processes that occur following injury to the CNS. In particular, astrocytic expression of cytokines and growth factors in the injured CNS has been well reviewed (2). Recently a few studies have detected the presence of chemokines in astrocytes following traumatic brain injury (3,4). These studies have suggested that chemokines may represent a promising target for future therapy of inflammatory conditions. This review summarizes the events that occur in traumatic brain injury and discusses the roles of resident and non-resident cells in the expression of growth factors, cytokines and chemokines in the injured CNS.
Similar content being viewed by others
REFERENCES
Ransohoff, R. M., Glabinski, A., and Tani, M. 1996. Chemokines in immune-mediated inflammation of the central nervous system. Cytokines and Growth Factor Reviews 7:35-46.
Rudge, J. S. 1993. Astrocyte-Derived Neurotrophic Factors. In: S. Murphy, ed., Astrocytes: Pharmacology and Function, Academic Press, Inc., San Diego, pp. 267-305.
Berman, J. W., Guida M. P., Warren J., Amat J., Brosnan, C.F. 1996. Localization of monocyte chemoattractant peptide-1 expression in the central nervous system in experimental autoimmune encephalomyelitis and trauma in the rat. J. Immunol 156:3017-23.
Glabinski, A. R., Balasingam, V., Tani, M., Kunkel, S. L., Strieter, R. M., Yong, V. W., Ransohoff, R. M. 1996. Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. J. Immunol 156:4363-4368.
Logan, A., and Berry, M. 1993. Transforming growth factor β1 & basic fibroblast growth factor in the injured CNS. Trends Pharm Sci 14:337-343.
Lotan, M., and Schwartz, M. 1994. Cross talk between the immune system and the nervous system in response to injury: implications for regeneration. FASEB J. 8:1026-1033.
Eng, L. F., and Ghirnikar, R.S. 1994. GFAP and astrogliosis. Brain Pathol 4:229-237.
Eddleston, M., and Mucke, L. 1993. Molecular profile of reactive astrocytes, implications for their role in neurologic diseases. Neuroscience 54:15-36.
Hopkins, S. J., and Rothwell, N. J. 1995. Cytokines and the nervous system. I: Expression and recognition. Trends in Neurosci 18:83-8.
Benveniste, E. N. 1993. Astrocyte-microglia interactions. In: Murphy S (ed): “Astrocytes: Pharmacology and Function.” San Diego: Academic Press, Inc., pp. 355-382.
Balasingam, V., and Yong, V. W. 1996. Attenuation of Astroglial Reactivity by Interleukin-10. J. Neurosci. 16:2945-2955.
DeKosky, S. T., Styren, S. D., O'Malley, M. E., Gross, J. R., Kochanek, P., Marion, D., Evans, C. H., and Robbins, P. D. 1996. Interleukin-1 receptor antagonist suppresses neurotrophin response in injured rat brain. Annals Neurol 39:123-7.
Kiefer, R., Lindholm, D., and Kreutzberg, G. W. 1993. Interleukin-6 and transforming growth factor-beta 1 mRNAs are induced in rat facial nucleus following motoneuron axotomy. European J Neurosci 5:775-781.
Sawada, M., Suzumura, A., and Marunouchi, T. 1995. Cytokine network in the central nervous system and its roles in growth and differentiation of glial and neuronal cells. International J Dev Neurosci 13:253-64.
Eng, L. F., Ghirnikar, R. S., and Lee, Y. L. 1996. Inflammation in EAE: Role of Chemokine/Cytokine expression by resident and infiltrating cells. Neurochem Res 21:511-525.
Balasingam, V., Tejada-Berges, T., Wright, E., Bouckova, R., and Yong, V. W. 1994. Reactive astrogliosis in the neonatal mouse brain and its modulation by cytokines. J Neurosci 14:846-56.
Nieto-Sampedro, M., Lewis, E. R., Cotman, C. W., Manthorpe, M., Skaper, S. D., Barbin, G., Longo, F. M., and Varon, S. 1982. Brain injury causes a time-dependent increase in neuronotrophic activity at the lesion site. Science 217:860-861.
Yong, V.W. 1996. Cytokines, Astrogliosis and Neurotrophism following CNS Trauma. In Cytokines and the CNS. RM Ransohoff, En Benveniste (eds) CRC Press Inc. pp 309.
Logan, A., Frautschy S. A., Gonzalez, A. M., Sporn, M. B., and Baird, A. 1992. Enhanced expression of transforming growth factor β-1 in the rat brain after a localized cerebral injury. Brain Res 587:216-25.
Finklestein, S. P., Apostolides, P. J., Caday, C. G., Prosser, J., Philips, M. F., and Klagsbrun, M. 1988. Increased basic fibroblast growth factor (bFGF) immunoreactivity at the site of focal brain wounds. Brain Res 460:253-9.
Hou, Y. J., Yu, A. C. H., Garcia, J. M. R. Z., Aotaki-Keen, A., Lee, Y. L., Eng, L. F., Hjelmeland, L. J., and Menon, V. K. 1995. Astrogliosis in culture: IV. Effects of basic fibroblast growth factor. J. Neurosci. Res. 40:359-370.
Johns, L. D., Flanders, K. C., Raanges, G. E., and Sriram, S. Successful treatment of experimental autoimmune encephalomyelitis with transforming growth factor-β-1 J. Immunol. (1991) 147:11792-1796.
Racke, M. K., Dhib-Jalbut, S., Cannella, B., Albert, P. S., Raine, C. S., and McFarlin, D. E. 1991. Prevention and treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor beta 1. J. Immunol., 146:3012-3017.
Racke, M. K., Sriram, S., Carlino, J., Cannella, B., Raine, C. S., and McFarlin, D. E. 1993. Long term treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor-beta 2. J. Neuroimmunol., 46:175-183.
Santambrogio, L., Hochwald, G. U., Saxena, B., Lev, C. H., Martz, J. E., Carlino, J. A., Ruddle, N. H., Palladino, M. A., Gold, L. I., and Thorbecke, G. J. 1993. Studies on the mechanisms by which transforming growth factor-β (TGF-β) protects against allergic encephalomyelitis. J. Immunol. 153:1116-1127.
Glabinski, A. R., Tani, M., Aras, S., Stoler, M. H., Tuohy, V. K., and Ransohoff, R. M. 1995. Regulation and function of central nervous system chemokines. Int J Dev Neurosci 13:153-65.
Tanaka, Y., Adams, D. H., Hubscher, S., Hirano, H., Siebenlist, U., and Shaw, S. 1993. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1 beta. Nature, 361:79-82.
Murphy, P. 1994. The molecular biology of leukocyte chemoattractant receptors. Ann Rev. Immunol. 12:593-633.
Horuk, R. 1994. Molecular properties of the chemokine receptor family. Trends in Pharmacol. Sci. 15:159-165.
Ben-Baruch, A., Michiel, D. F., Oppenheim, J. J. 1995. Signals and receptors involved in recruitment of inflammatory cells. J. Biol. Chem. 270:11703-11706.
Van Damme, J., Rampart, M., Conings, R., Decock, B., Van Osselaer, N., Willems, J., and Billiau, A. 1990. The neutrophil-activating proteins interleukin 8 and beta thromboglobulin: in vitro and in vivo comparison of NH2 terminally processed forms. European J. Immunol. 20:2113-2118.
Loetscher, M., Geiser, T., O'Reilly, T., Zwahlen, R., Baggiolini, M., and Moser, B. 1994. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J. Biol. Chem. 269:232-237.
Nakagawa, H., Komorita, N., Shibata, F., Ikesue, A., Konishi, K., Fujioka, M., Kato, H. 1994. Identification of cytokine induced neutrophil chemoattractants (CINC), rat GRO/CINC-2 alpha and CINC-2 beta, produced by granulation tissue in culture: purification, complete amino acid sequences and characterization. Biochemical J. 301:545-550.
Wu, X., Wittwer, A. J., Carr, L. S., Crippes, B. A., DeLarco, J. E., and Lefkowith, J. B. 1994. Cytokine-induced neutrophil chemoattractant mediates neutrophil influx in immune complex glomerulonephritis in rat. J. Clinical Investigation 94:337-344.
Smoller, B. R., and Krueger, J. 1991. Detection of cytokine-induced protein gamma-immune protein-10 (gamma IP10) in atypical melanocytic proliferations. J. American Academy of Dermatology, 25:627-631.
Minty, A., Chalon, P., Guillemot, J. C., Kaghad, M., Liauzun, P., Magazin, M., Miloux, B., Minty, C., Raymond, P., Vita, N. et al 1993. Molecular cloning of the MCP-3 chemokine gene and regulation of its expression. Eur Cytokine Network 4:99-110.
Schmouder, R. L., Strieter, R. M., and Kunkel, S. L. 1993. Interferon gamma regulation of human renal cortical epithelial cell-derived monocyte chemotactic peptide-1. Kidney Int 44:43-49.
Kuna. P., Reddigari, S. R., Schall, T. J., Rucinski, D., Sadick, M., and Kaplan, A. P. 1993. Characterization of the human basophil response to cytokines, growth factors, and histamine releasing factors of the intercrine/chemokine family. J Immunol 150:1932-43.
Kawahara, R. S., Deng, Z. W., Denkinger, D. J., and Deuel, T. F. 1994. Role of serine/threonine protein kinases in the induction of JE, a platelet derived growth factor inducible gene. Biochem. Biophys. Res. Comm. 203:1815-20.
Dahinden, C. A., Geiser, T., Brunner, T., von Tscharner, V., Caput, D., Ferrara, P., Minty, A., and Baggiolini, M. 1994. Monocyte chemotactic protein 3 is a most effective basophil-and eosinophil-activating chemokine. J. Exp. Med. 179:751-756.
Luo, Y., Laning, J., and Dorf, M. E. 1993. Serologic analysis of a murine chemokine, TCA3. J. Immunol. 150:971-979.
Wilson, S. D., Billings, P. R., D'Eustachio, P., Fournier, R. E., Geissler, E., Lalley, P. A., Burd, P. R., Housman, D. E., Taylor, B. A., and Dorf, M. E. 1990. Clustering of cytokine genes on mouse chromosome 11. J Exp Med 171:1301-1314.
Taub, D. D., Conlon, K., Lloyd, A. R., Oppenheim, J. J., and Kelvin, D.J. 1993. Preferential migration of activated CD4+ and CD8+ T cells in resonse to MIP-1 alpha and MIP-1 beta. Science 260:355-358.
Neote, K., DiGregorio, D., Mak, J.Y., Horuk, R., and Schall, T.J. 1993. Molecular cloning, functional expression, and signaling characteristics of a C-C chemokine receptor. Cell 72:415-425.
Schall, T. J., Bacon, K., Toy, K. J., and Goeddel, D. V. 1990. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature 47:669-71.
Godiska, R., Chantry, D., Dietsch, G. N., and Gray, P. W. 1995. Chemokine expression in murine experimental allergic encephalomyelitis. J. Neuroimmunol. 58:167-176.
Rathanaswami, P., Hachicha, M., Sadick, M., Schall, T. J., and McColl, S. R. 1993. Expression of the cytokine RANTES in human rheumatoid synovial fibroblasts. Differential regulation of RANTES and interleukin-8 genes by inflammatory cytokines. J. Biol. Chem. 268:5834-5839.
Bischoff, S. C., Krieger, M., Brunner, T., Rot, A., von Tscharner, V., Baggiolini, M., and Dahinden, C. A. 1993. RANTES and related chemokines activate human basophil granulocytes through different G protein-coupled receptors. Euro. J. Immunol. 23:761-767.
Meurer, R., Van Riper, G., Feeney, W., Cunningham, P., Hora, D. Jr, Springer, M. S., McIntyre, D. E., and Rosen, H. 1993. Formation of eosinophilic and monocytic intradermal inflammatory sites in the dog by injection of human RANTES but not human monocyte chemoattractant protein 1, human macrophage inflammatory protein 1 alpha or human interleukin 8. J. Exp. Med. 178:1913-1921.
Tani, M., and Ransohoff, R. M. 1994. Do chemokines mediate inflammatory cell invasion of the central nervous system parenchyma? Brain Pathol. 4:135-143.
Ransohoff, R. M., Hamilton, T. A., Tani, M., Stoler, M. H., Shick, H. E., Major, J. A., Estes, M. L., Thomas, D. M., and Tuohy, V. K. 1993. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. Faseb J. 7:592-600.
Brosnan, C. F., Shafit-Zagardo, B., Aquino, D. A., and Berman, J. W. 1993. Expression of monocyte/macrophage growth factors and receptors in the CNS. Adv. Neurol. 59:349-361.
Hulkower, K., Brosnan, C. F., Aquino, D. A., Cammer, W., Kulshrestha, S., Guida, M. P., Rapoport, D. A., and Berman, J. W. 1993. Expression of CSF-1, c-fms, & MCP-1 in the CNS of rats with EAE. J. Immunol. 150:2525-2533.
Barna, B. P., Pettay, J., Barnett, G. H., Zhou, P., Iwasaki, K., and Estes, M. L. 1994. Regulation of monocyte chemoattractant protein-1 expression in adult human non-neoplastic astrocytes is sensitive to tumor necrosis factor (TNF) or antibody to the 55-kDa TNF receptor. J. Neuroimmunol. 50:101-7.
Hurwitz, A. A., Lyman, W. D., and Berman, J. W. 1995. Tumor necrosis factor α and transforming growth factor β upregulate astrocyte expression of monocyte chemoattractant protein-1. J. Neuroimmunol. 57:193-8.
Karpus, W. J., Lukas, N. W., McRae, B. L., Strieter, R. M., Kunkel, S. L., and Miller, S. D. 1995. An important role for the chemokine macrophage inflammatory protein-1 α in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J. Immunol. 155:5003-10.
Murphy, G. M. Jr., Jia, X.-C., Song, Y., Ong, E., Shrivastava, R., Bocchini, V., Lee, Y. L., and Eng, L. F. 1995. Macrophage inflammatory protein 1-α mRNA expression in an immortalized microglial cell line and cortical astrocyte cultures. J. Neurosci. Res. 40:755-763.
Noe, K. H., Fisher, S. N., Dhib-Jalbut, S. S., and Shin, M. L. 1996. Induction of cytokine RANTES by virus in astrocytes. J. Neurochem. 66:Supp S71D.
Mathewson, A. J., and Berry, M. 1985. Observations on the astrocyte response to a cerebral stab wound in adult rats. Brain Res 327:61-69.
Topp, K. S., Faddis, B. T., and Vijayan, V. K. 1989. Trauma induced proliferation of astrocytes in the brains of young and aged rats. Glia 2:201-211.
Vijayan, V. K., Lee, Y. L., and Eng, L. F. 1990. Increase in glial fibrillary acidic protein following neural trauma. Mol. Chem. Neuropathol. 13:107-118.
Norton, W. T., Aquino, D. A., Hozumi, I., Chiu, F. C., and Brosnan C. F. 1992. Quantitative aspects of reactive gliosis: a review. Neurochemical Res. 17:877-885.
Ghirnikar, R. S., Lee, Y. L., He, T. R., and Eng, L. F., 1996. Chemokine expression in rat stab wound brain injury. J. Neuroscience Research, 46:727-733.
Maxwell, W. L., Follows, R, Ashhurst, D. E., and Berry, M. 1990. The responses of the cerebral hemisphere of the rat to injury. I. The mature rat. Phil. Trans. R. Soc. Lond. B. 328:479-500.
Loo, D. T., Althoen, M. C., and Cotman, C. W. 1995. Differentiation of serum-free mouse embryo cells into astrocytes is accompanied by induction of glutamine synthetase activity. J. Neurosci. Res. 42:184-91.
D'Amelio, F., Eng, L. F., and Gibbs, M. A. 1990. Glutamine synthetase immunoreactivity is present in oligodendroglia of various regions of the central nervous system [see comments]. Glia 3:335-41.
Pixley, S. R., and de Villis, J. 1984. Transition between immature radial glia and mature astrocytes studied with a monoclonal antibody to vimentin. Dev. Brain Res. 15:201-209.
Dahl, D., Strocchi, P., and Bignami, A. 1982. Vimentin in the central nervous system. A study of the mesenchymal type intermediate filament protein in Wallerian degeneration and in postnatal rat development by two-dimensional gel electrophoresis. Differentiation 22:185-190.
Schiffer, D., Giordana, M. T., Migheli, A., Giaccone, G., Pezzotta, S., and Mauro, A. 1986. Glial fibrillary acidic protein and vimentin in the experimental glial reaction of the rat brain. Brain Res. 374:110-8.
Schiffer, D., Giordana, M. T., Cavalla, P., Vigliani, M. C., and Attanasio, A. 1993. Immunohistochemistry of glial reaction after injury in the rats: Double stainings and markers of cell proliferation. Int. J. Dev. Neurosci. 11:269-280.
Takamiya, Y., Kohsaka, S., Toya, S., Otani, M., and Tsukada, Y. 1988. Immunohistochemical studies on the proliferation of reactive astrocytes and the expression of cytoskeletal proteins following brain injury in rats. Brain Res 466:201-10.
Graeber, M. B., Streit, W. J., and Kreutzberg, G. W. 1988b. The microglial cytoskeleton: vimentin is localized within activated cells in situ. J. Neurocytol. 17:573-80.
Schnitzer, J., Franke, W. W., and Schachner, M. 1981. Immunocytochemical demonstration of vimentin in astrocytes and ependymal cells of developing and adult mouse nervous system. J Cell Biol 90:435-47.
Stichel, C. C., and Muller, H. W. 1994. Extensive and long-lasting changes of glial cells following transection of the postcommissural fornix in the adult rat. Glia 10:89-100.
Calvo, J. L., Carbonell, A. L., and Boya, J. 1991. Co-expression of glial fibrillary acidic protein and vimentin in reactive astrocytes following brain injury in rats. Brain Res 566:333-6.
Fernaud-Espinosa, I., Nieto-Sampedro, M., and Bovolenta, P. 1993. Differential activation of microglia and astrocytes in aniso-and isomorphic gliotic tissue. Glia, 8(4):277-91.
Perry, V. H., Matyszak, M. K., and Fearn, S. 1993. Altered antigen expression of microglia in the aged rodent CNS. Glia 7:60-7.
Moreno-Flares, M. T., Bovolenta, P., and Nieto-Sampedro, M. 1993. Polymorphonuclear Leukocytes in Brain Parenchyma After Injury and their interaction with purified astrocytes in culture. Glia 7:146-157.
Holmin, S., Mathiesen, T., Shetye, J., and Biberfeld, P. 1995. Intracerebral inflammatory response to experimental brain contusion. Acta Neurochirurgica 132:110-9.
Ling, E. A., and Wong, W. C. 1993. The original and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia 7:9-18.
Jacque, C., Tchelingerian, J. L., 1994. [New concepts on the role of cytokines in the central nervous system]. Revue Neurologique 150:748-56.
Benveniste, E. N., and Benos, D. J. 1995. TNF-α-and IFN-gamma-mediated signal transduction pathways: effects on glial cell gene expression and function. Faseb Journal 9:1577-84.
Cheng, B., Christakos, S., and Mattson, M. P. 1994. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron 12:139-53.
Kahn, M. A., Ellison, J. A., Speight, G. H., and De Vellis, J. 1995. CNTF regulation of astrogliosis and the activation of microglia in the developing rat central nervous system. Brain Res. 685:55-67.
Eng, L. F., Lee, Y. L., and Yu, A. C. H. 1994a. Gene expression in a mechanical injury model. Trans. Am. Soc. Neurochem. 25:235.
Norris, J. G., Tang, L. P., Sparacio, S. M., and Benveniste, E. N. 1994. Signal transduction pathways mediating astrocyte IL-6 induction by IL-1 β and tumor necrosis factor-α. J. Immunol. 152:841-50.
Woodroofe, M. N., Sarna, G. S., Wadhwa, M., Hayes, G. M., Loughlin, A. J., Tinker, A., and Cuzner, M. L. 1991. Detection of interleukin-1 and interleukin-6 in adult rat brain, following mechanical injury, by in vivo microdialysis: evidence of a role for microglia in cytokine production. J. Neuroimmunol. 33:227-236.
Taupin, V., Toulmond, S., Serrano, A., Beavides, J., and Zavala, F. 1993. Increase in IL-6, IL-1 and TNF levels in the rat brain following traumatic lesion. J. Neuroimmunol. 42:177-186.
Chiang, C. S., Stalder, A., Samimi, A., and Campbell, I. L. 1994. Reactive gliosis as a consequence of interleukin-6 expression in the brain: studies in transgenic mice. Dev. Neurosci. 16:212-21.
Brett, F. M., Mizisin, A. P., Powell, H. C., and Campbell, I. L. 1995. Evolution of neuropathologic abnormalities associated with blood-brain barrier breakdown in transgenic mice expressing interleukin-6 in astrocytes. J. Neuropathol. and Exp. Neurol. 54:766-775.
Hariri, R. J., Chang, V. A., Barie, P. S., Wang, R. S., Sharif, S. F., and Ghajar, J. B. 1994. Traumatic injury induces interleukin-6 production by human astrocytes. Brain Res. 636:139-42.
Benveniste, E. N., Sparacio, S. M., Norris, J. G., Grenett, H. E., Fuller, G. M. 1990. Induction and regulation of interleukin-6 gene expression in rat astrocytes. J. Neuroimmunol. 30:201-12.
Murphy, G. M. Jr., Jia, X.-C., Yu, A. C. H., Lee, Y. L., Tinklenberg, J. R., and Eng, L. F. 1993. Reverse transcription and polymerase chain reaction technique for quantification of mRNA in primary astrocyte cultures. J. Neurosci. Res. 35:643-651.
Finch, C. E., Laping, N. J., Morgan, T. E., Nichols, N. R., and Pasinetti, G. M. 1993. TGF-β 1 is an organizer of responses to neurodegeneration. J. Cell Biochem. 53:314-22.
Pasinetti, G. M., Nichols, N. R., Tocco, G., and Morgan T. 1993. Transforming growth factor-β1 and fibronectin messenger RNA in rat brain: responses to injury and cell type localization. Neuroscience 54:893-907.
Wahl, S. M., Allen, J. B., McCartney-Francis, N., Morganti-Kossman, T., Ellingsworth, L., Mai, U. E., Mergenhagen, S. E., and Orenstein, J. M. 1991. Macrophage-and astrocyte-derived transforming growth factor β as a mediator of central nervous system dysfunction in acquired immune deficiency syndrome. J. Exp. Med. 173:981-91.
O'Brien, M. F., Lenke, L. G., Lou, J., Bridwell, K. H., and Joyce, M. E. 1994. Astrocyte response and transforming growth factor-β localization in acute spinal cord injury. Spine 19:2321-2330.
Kiefer, R., Lindholm, D., and Kreutzberg, G. W. 1993. Interleukin-6 and transforming growth factor-beta 1 mRNAs are induced in rat facial nucleus following motoneuron axotomy. European J. Neurosci. 5:775-781.
Vergeli, M., Mazzanti, B., Ballerini, C., Gran, B., Amaducci, L., and Massacesi, L. 1995. Transforming growth factor-β 1 inhibits the proliferation of rat astrocytes induced by serum and growth factors. J. Neurosci. Res. 40:127-33.
Logan, A., Berry, M., Gonzalez, A. M., Frautschy, S. A., Sporn, M. B., and Baird, A. (1994): Effects of transforming growth factor β 1 on scar production in the injured central nervous system of the rat. Eur. J. Neurosci. 6:355-63.
Galbreath, E., Kim, S. J., Park-K, Brenner, M., and Messing, A. (1995): Overexpression of TGF-β 1 in the central nervous system of transgenic mice results in hydrocephalus. J. Neuropathol. Exp. Neurol. 54:339-49.
Lefer, A. M., (1991): Mechanisms of the protective effects of transforming growth factor-β in reperfusion injury. Biochemical Pharmacology 42:1323-7.
Frautschy, S. A., Walicke, P. A., and Baird, A. (1991): Localization of basic fibroblast growth factor and its mRNA after CNS injury. Brain Res. 553:291-299.
Gomez-Pinilla, F., Lee, J. W. K., and Cotman, C. W. (1992): Basic FGF in adult rat brain: cellular distribution and response to entorhinal lesion and fimbria-fornix transection. J. Neurosci. 12:345-355.
Woodward, W. R., Nishi, R., Meshul, C. K., Williams, T. E., Coulombe, M., and Eckenstein, F. P. (1992): Nuclear and cytoplasmic localization of basic fibroblast growth factor in astrocytes and CA2 hippocampal neurons. J. Neurosci. 12:142-152.
Vijayan, V. K., Lee, Y. L., and Eng, L. F. (1993) Immunohistochemical localization of basic fibroblast growth factor in cultured rat astrocytes and oligodendrocytes. Int. J. Dev. Neurosci. 11:257-267.
Neugarten, J., Feith, G. W., Assmann, K. J., Shan, Z., Stanley, E. R., and Schlondorff, D. 1995. Role of macrophages and colony-stimulating factor-1 in murine antiglomerular basement membrane glomerulonephritis. J. Am. Soc. Nephr. 5:1903-1909.
VanOtteran, G. M., Strieter, R. M., Kunke, S. L., Paine R 3rd, Greenberger, M. J., Danforth, J. M., Burdick, M. D., and Standiford, T. J. (1995). Compartmentalized expression of RANTES in a murine model of exdotoxemia. J. Immunol. 154:1900-8.
Seebach, J., Bartholdi, D., Frei, K., Spanaus, K. S., Ferrero, E., Widmer, U., Isenmann, S., Strieter, R. M., Schwab, M., Pfister H., et al. 1995. Experimental Listeria meningoencephalitis Macrophage inflammatory protein-1 α and-2 are produced intrathecally and mediate chemotactic activity in cerebrospinal fluid of infected mice. J. Immunol. 155:4367-75.
Merrill, J. E., and Benveniste, E. N. (1996): Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci. 19:331-338.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ghirnikar, R.S., Lee, Y.L. & Eng, L.F. Inflammation in Traumatic Brain Injury: Role of Cytokines and Chemokines. Neurochem Res 23, 329–340 (1998). https://doi.org/10.1023/A:1022453332560
Issue Date:
DOI: https://doi.org/10.1023/A:1022453332560